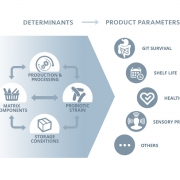
The FDA’s view on the term probiotics, part 1
By James Heimbach, Ph.D., F.A.C.N., JHEIMBACH LLC, Port Royal, VA

James Heimbach, food and nutrition regulatory consultant
Over the past 20 years as a food and nutrition regulatory consultant, I have filed about 40 GRAS notices with the United States Food and Drug Administration (FDA), including 15 strains of probiotic bacteria and 5 prebiotics. This fall I submitted notices dealing with 4 strains of bacteria and on January 16 received a telephone call from FDA that surprised me and initially infuriated me, but which I have come to understand.
The essence of the call was that FDA was declining to file my probiotic notices because the notices had identified the subject bacteria as “probiotics” or “probiotic bacteria.” FDA suggested that I resubmit without calling the subject microorganisms “probiotics.”
As I said, I was surprised and frustrated, and I still would prefer that when FDA makes a policy swerve they would do it in a way that does not make extra work for me and delay my clients’ ability to get to market in a timely manner.
What I have had to do here is remove my advocate’s hat and put on my regulator’s hat. (I worked for FDA for a decade . . . long ago [1978 to 1988], but I still remember how to think like a regulator.) And here is the issue. Recall that GRAS is concerned with safety, not efficacy (generally recognized as safe, or GRAS), and the information provided in a GRAS notice is focused on safety (although benefits may be more-or-less incidentally covered). The reviewers at FDA are charged with assessing whether the notice provides an adequate basis to conclude that there is a reasonable certainty that no harm will result from the intended use. They are not charged, and they are not equipped, to evaluate what benefits ingestion of the substance or microorganism might provide. So they are not in a position to say whether the subject microorganism will “confer a health benefit on the host,” which is to say, they are not in a position to say whether or not it may be regarded as a probiotic. Remember, probiotics are defined as live microorganisms that, when administered in adequate amounts, confer a health benefit on the host (Hill et al. 2014).
Given that the FDA reviewers cannot say whether the notified microorganism is rightly called a probiotic, they are reluctant to sign off that they have no questions about a notice that calls it one. Regulatory agencies have to be careful; things sometimes come back to haunt them. Those who have been following FDA’s GRAS-notice response letters for a couple of decades will be aware that the agency is putting more and more disclaimers into the letters—about standards of identity, about potential labeling issues, about benefits shown in clinical trials, and about Section 201(II) of the FD&C Act.

One concern that FDA likely has is that if some issue comes up in the future regarding a claim made for benefits from use of a product containing the subject bacterium, someone may make the argument that FDA had accepted that the strain is indeed a probiotic and so it presumably confers probiotic benefits. In the case of probiotics, there are also some internal FDA politics. As ISAPP meeting attendees may already be aware, FDA’s Center for Biologics Evaluation and Research (CBER) would like to claim jurisdiction over all administration of live microorganisms, and the Center for Food Safety and Applied Nutrition (CFSAN) does not seem willing to have a confrontation.
I suspect that a similar situation obtains with the term “prebiotic.” Although I have filed a number of GRAS notices for prebiotics, they haven’t been called that; they have been called fructooligosaccharides, or tamarind seed polysaccharide, or polydextrose, or 2’-O-fucosyllactose. I don’t know how FDA would respond if a GRAS determination were filed with the substance labeled as a prebiotic.
So, I’ve decided that my sympathies lie with FDA. Until and unless a microorganism has been confirmed by competent authority to have probiotic properties when used as intended in a GRAS notice, FDA is probably correct in rejecting its right to be labeled a probiotic. If it’s any consolation, this new position by the FDA has its origin in their acknowledgment of the official scientific definition of the word “probiotic”.

When Mary Ellen Sanders (ISAPP’s Executive Science Officer) reviewed my first draft of this note, she asked what I had in mind by “competent authority,” to which I don’t have a good answer at the present time except to insist that it is not FDA’s Division of GRAS Notice Review. Thirty years ago, when I was at FDA, I was in the Office of Food Science and Nutrition, and that office was charged with making determinations of that type (although I don’t recall anything about probiotics coming before us). But FDA no longer has such an office. Until it does, or until it agrees on another source of authority on designation of microorganisms as non-CBER-domain probiotics, I suspect that CFSAN will continue to be very cautious in this area.
Read part 2 of this blog series here.