Expert Panel at ISAPP Annual Meeting Addresses Probiotic Use for Premature Infants
By Marla Cunningham, ISAPP Executive Director
The use of probiotics in premature infants has been highly topical in recent months. Probiotic use for the prevention of necrotising enterocolitis (NEC) in preterm infants has been studied in over 65 randomised clinical trials, with systematic reviews showing significant reductions in NEC as well as all cause mortality. However, the application of probiotics is not without risk – in vulnerable populations such as preterm infants, the translocation of probiotic bacteria into the bloodstream is a rare but documented occurrence. While probiotic bacteraemia is usually highly treatable with antibiotics, some isolated case reports of fatalities over the years have created significant concern. One such recent incident resulted in an FDA warning letter in September 2023 to all US healthcare practitioners, amongst other warning letters issued to companies for marketing breaches. The healthcare provider letter warned about the risk of probiotic products in premature infants and reminded clinicians that the recommendation of any non-approved products for disease prevention, such as NEC, must be conducted under an investigational new drug (IND) application. The prohibitive nature of IND applications in conjunction with the liability risk inherent in prescribing under the shadow of an FDA warning letter has severely limited the prescribing of probiotics in US neonatal intensive care units (NICUs).
While recent US events have brought this issue to the forefront, evidence gaps and disparate clinical implementation rates are challenges that exist across the globe. To further explore this issue, ISAPP held a panel discussion at the 2024 ISAPP Annual Scientific meeting in Cork, Ireland. The panel featured seven experts sharing their unique perspectives on this complex issue, covering scientific, clinical, regulatory, industry, and patient family viewpoints.
Evaluating evidence for risk versus benefit
Dr. Geoffrey Preidis, MD PHD, paediatric and neonatal gastroenterologist at Baylor College of Medicine, set the scene with an overview of the current evidence base on probiotics for prevention of NEC in preterm infants. Covering recent meta analyses and systematic reviews (AGA 2020, Cochrane 2023), he explored the strength and quality of evidence for risk and benefit, and highlighted recommendations and concerns raised by clinical societies. While the American Gastroenterological Association (AGA) made a positive conditional recommendation for probiotic use for the prevention of NEC in premature infants supported by moderate/high quality evidence, the American Academy of Pediatrics (AAP), while acknowledging discretionary use in certain units, recommended against universal use in NICUs due to safety concerns. Exploring the specific safety concerns, Dr Preidis highlighted that sepsis risk due to contamination of probiotics with pathogenic organisms was a matter requiring ongoing attention and could approach zero with continued efforts, as outlined in a paper he co-authored in JAMA Pediatrics about optimising product standards. While probiotic organism-induced sepsis remains a risk with the administration of live microbes, Dr Preidis’ assessment of risk:benefit calculations remained strongly in favour of benefit. Reported number needed to treat with prophylactic probiotic administration is 50 infants to prevent 1 death, and while probiotic sepsis-induced mortality is difficult to accurately estimate, conservative calculations (likely overestimating risk) suggest 1:8000.
Continuing data collection
 Dr. Mark Underwood MD, neonatologist at Providence Sacred Heart Medical Center in Washington state, described two large ongoing trials of probiotics for NEC prevention, as well as the efforts to track preterm infant outcomes in the US before and after the FDA warning letter. He also outlined the current litigation environment in the US, with a surge in lawsuits addressing infant formula administration and NEC risk further intensifying the already litigious environment in the NICU. This situation contributes to why US hospitals are unwilling to allow probiotic administration in the NICU after the FDA warning. He highlighted key research priorities for the field, and explored the benefits of a cluster-randomized (per NICU) crossover trial with early gestational age (<32 weeks) infants, to overcome multiple NICU-specific confounders. Dr. Underwood also raised the question of clinical equipoise for ethical study design – with data on over 15,000 infants and significant effect sizes, does equipoise exist to conduct further placebo-controlled trials with infants?
Dr. Mark Underwood MD, neonatologist at Providence Sacred Heart Medical Center in Washington state, described two large ongoing trials of probiotics for NEC prevention, as well as the efforts to track preterm infant outcomes in the US before and after the FDA warning letter. He also outlined the current litigation environment in the US, with a surge in lawsuits addressing infant formula administration and NEC risk further intensifying the already litigious environment in the NICU. This situation contributes to why US hospitals are unwilling to allow probiotic administration in the NICU after the FDA warning. He highlighted key research priorities for the field, and explored the benefits of a cluster-randomized (per NICU) crossover trial with early gestational age (<32 weeks) infants, to overcome multiple NICU-specific confounders. Dr. Underwood also raised the question of clinical equipoise for ethical study design – with data on over 15,000 infants and significant effect sizes, does equipoise exist to conduct further placebo-controlled trials with infants?
Hearing European and UK perspectives
Prof Hania Szajewska MD PhD, chair of Paediatrics at the Medical University of Warsaw, explored key points of the 2023 European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) statement, produced following the FDA warning. The point was made that while a move to pharmaceutical grade products could reduce contamination risk, probiotic-induced sepsis rates were unlikely to be mitigated by such a strategy, and noted the risk for lives lost of abandoning currently available treatments in the short term. The statement also emphasised the crucial role of parents in decision making about infant care.
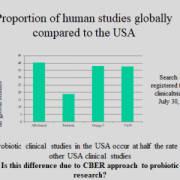
While rates of probiotic usage in US NICUs have plummeted to an estimated <3% since the FDA warning, usage rates across NICUs in the UK were estimated at around 40% of NICUs in 2022, and are not known to have been reduced. Discussing UK implementation data and frameworks for use, Dr Janet Berrington MD, neonatal consultant in Newcastle, UK, also highlighted further research priorities for the field, including the limited data on probiotic use in <28 week/<1000g infants. She called for a focus on improved data collection on probiotic use and standardised diagnostic and outcome reporting for clinical studies as well as within national registries. Sharing work from her own studies, Dr Berrington highlighted the utility of pre-trial understanding of the gut microbiome impact of probiotics, where improved maturation of the microbiome in response to a given probiotic was predictive of benefits in clinical studies.
Prioritising shared decision making
Sharing perspectives from patient families of NEC sufferers, Marie Spruce, chair of the charity organisation NEC UK, highlighted parent concerns about lack of information about probiotic treatment options, possible risks and benefits, and the level of parent consultation in decision-making during NICU care of infants. Ultimately, she highlighted, parents live with the consequences of decisions made in hospital, and she emphasised the importance of ensuring parent views are sought within critical decision making windows.
Understanding the US legal environment
 Understanding the legal, regulatory and practical barriers to improved utilisation in NICUs, across the US and globally, was deemed critical to moving forward. Prof Diane Hoffmann of the University of Maryland explored current regulatory and legal constraints within the US environment, as well as potential paths forward for probiotic use, including medical food, drug and biologic pathways, and accelerated/expanded access for probiotic products undergoing IND approval. Questions were raised about the scope of regulatory influence over clinician autonomy in the practice of medicine – an area where different perspectives exist and where litigation remains a risk.
Understanding the legal, regulatory and practical barriers to improved utilisation in NICUs, across the US and globally, was deemed critical to moving forward. Prof Diane Hoffmann of the University of Maryland explored current regulatory and legal constraints within the US environment, as well as potential paths forward for probiotic use, including medical food, drug and biologic pathways, and accelerated/expanded access for probiotic products undergoing IND approval. Questions were raised about the scope of regulatory influence over clinician autonomy in the practice of medicine – an area where different perspectives exist and where litigation remains a risk.
Incorporating solutions from industry
Providing an industry perspective, Dr Greg Leyer PhD conveyed the capabilities of industry to support better infant health within manufacturing and regulatory constraints. Dr Leyer presented on the possibilities for improved product quality through manufacturing and testing standard initiatives, transparency and third party verification, as well as post-market surveillance initiatives. He noted that companies are at a crossroads in decision-making around infant-focused product development, considering the risks and markets in the US and globally.
Identifying priorities for the future
Engaging with the panel during Q+A time, a number of audience members questioned where legal liability lies for a failure to treat, given the level of evidence in support of probiotic administration. Prof Hoffmann noted that in the current US context and given the FDA warning, a lawsuit claiming fault from a lack of probiotic administration would most likely not be successful. Other audience members commented on treatment rights for parents with children in NICUs without established probiotic use protocols. While panelists noted a lack of clarity in this area, a strategy sometimes employed by mothers expressing milk for their hospitalised infants was maternal consumption of probiotics. Some audience members questioned whether the lack of alignment on recommendations from professional medical societies may have influenced regulatory decision-making and whether better alignment on clinical guidelines should be a priority moving forward. Closing recommendations included ensuring that appropriate consideration of the large body of scientific evidence is paramount in regulatory and clinical decision making as well as prioritising parent education and consultation for truly informed decisions.
See here for the ISAPP statement on probiotic administration in premature infants, published after the FDA warning to healthcare professionals.