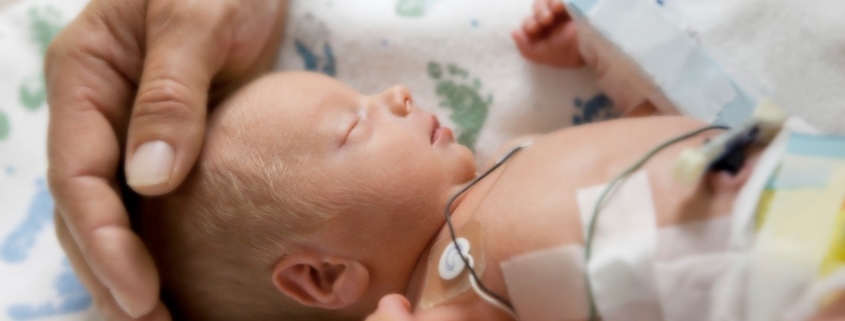
Probiotic Administration in Preterm Infants: Scientific Statement
Board of Directors, International Scientific Association for Probiotics and Prebiotics
in collaboration with
Dr. Geoffrey Preidis MD PhD, Pediatric Gastroenterology, Hepatology & Nutrition
Prof. Andi L Shane MD MPH MSc, Pediatric Infectious Diseases
A recent report of a fatality in an extremely premature infant recipient of a probiotic product has resulted in a warning letter from the United States Food & Drug Administration (FDA) to healthcare practitioners about probiotic supplementation in preterm infants and a warning letter to the probiotic product manufacturer.
Publicly available information suggests that this fatality was the direct consequence of bacteremia resulting from ingestion of the probiotic organism Bifidobacterium longum subsp. infantis delivered in medium chain triglyceride oil. This situation differs from case reports of adverse events that resulted from extrinsic probiotic product contamination (1, 2). This is an important distinction, as the potential risks and mitigation strategies differ between etiologies. As complete details of this most recent fatality have not been released, specific factors that may have contributed to the adverse outcome are unknown. However, it is worth considering the context of this case report within the broader literature available on probiotic use in this population, including the wealth of data available on sepsis incidence.
Evidence from systematic reviews
Premature infants, especially those of <32 weeks gestation and with a birth weight <1500 g, are a vulnerable population at significant risk of morbidity and mortality. Necrotizing enterocolitis (NEC) is highly prevalent (5-10% incidence) among very preterm infants, with mortality rates of 20-30% and high morbidity among survivors, including short gut syndrome, parenteral nutrition-associated liver disease, and neurocognitive delay.
A large body of literature exists on the use of probiotics in hospitalized preterm infants, with particular focus on the prevention of NEC. At least 85 randomised clinical trials (RCTs) (3) have been conducted to evaluate the use of probiotics in preterm infants for the prevention of diseases associated with prematurity, and a number of systematic reviews with meta-analyses have analysed these data in recent years. Most RCTs conducted in the neonatal intensive care unit (NICU) designate sepsis as one of the main outcome measures.
The most recent meta-analysis was published online October 2 in JAMA Pediatrics (3). This study included 106 trials on probiotic, prebiotic, synbiotic and lactoferrin interventions for either preterm infants <37 weeks and/or those with low birth weight (<2500 g). Administration of probiotics containing multiple strains were found to be most effective in the reduction of all-cause mortality (31% reduction), with a 62% decrease in incidence of severe NEC compared to placebo (moderate and high certainty evidence). Single strain probiotics combined with lactoferrin provided greatest efficacy in the reduction of late-onset sepsis incidence (67% risk reduction with moderate certainty evidence). It was noted that none of the included studies reported cases of probiotic-induced sepsis.
Other authors including groups from the Cochrane Collaboration, American Gastroenterological Association (AGA) and the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) have found similar results, and studies can be reviewed here:
No meta-analysis has attributed increased risk of sepsis to probiotic use in preterm infants – rather, in many cases a protective effect (or a trend toward protection) was reported. However, it is important to acknowledge the real but rare risk of probiotic-induced bacteremia in this population. In a recent review of case reports of probiotic-associated invasive infections in children, probiotic-induced bacteremia in premature infants were found to have resolved in most cases with use of effective antimicrobial therapy (4).
With data collected on over 10,000 preterm infants, substantial benefits demonstrated and a low level of risk identified, promise to improve outcomes in preterm infants who receive a probiotic product currently exists. Based on the evidence currently available, hospitals and NICUs across the globe have already adopted practices relating to probiotic use in preterm infants, some with significant health impacts (5, 6).
Risk benefit analysis and considerations for healthcare implementation
Further work needs to be done to support probiotic administration in the NICU. Collaborative efforts include recommendations for practical steps to improve probiotic product quality assurance specifically for NICU use, published in July 2023 in JAMA Pediatrics (7).
It is important to note that few (or possibly no) effective interventions are without an adverse event profile, and probiotics are no exception. Even food has a safety standard of reasonable certainty and on a regular basis, individuals suffer fatal foodborne infections. When considering the clinical indications for any intervention for an individual patient or a population of individuals, a thorough comparison of all available data on both the potential risks and the potential benefits is warranted.
The American Gastroenterological Association (8) and other major societies (including ESPGHAN and the World Gastroenterology Organisation) (9, 10) endorse probiotic products for the prevention of NEC among preterm low birth weight infants. The societies’ guidelines agree that the recommendation to use probiotics is conditional. Conditional recommendations are sensitive to patients’ values and preferences, and to the guideline panel’s perception of risk-benefit balance. However, the recent FDA letter does not acknowledge these recommendations and further, recommends against probiotic use in preterm infants despite the robust efficacy data. With interventions such as probiotic administration, ideally shared clinical decision-making with patient and clinician would ensue. Regulatory warnings inform the risk-benefit calculation but typically do not invalidate a clinical recommendation.
Summary
- Probiotic administration to preterm infants has been demonstrated to significantly reduce the risk of NEC, sepsis and death in large systematic reviews with meta-analyses.
- Meta-analyses have not identified significant adverse events or safety concerns, although rare case reports have documented sepsis attributed to probiotics.
- Stringent manufacturing standards are recommended for probiotics in vulnerable populations such as preterm infants.
- Standardized comprehensive safety reporting across probiotic intervention studies is needed, along with funding for the conduct of long term studies.
- The risks and benefits of probiotic administration should be considered in both the specific population and individual patients, with regulatory frameworks to enable implementation.
- More information about this fatality should be immediately released so healthcare professionals and researchers can learn from this experience and continue to provide optimal evidence-based patient care.
To inquire about expert academic physicians available for media comment, please contact ISAPP’s Executive Director, Marla Cunningham, at marla@nullisappscience.org
See also:
NEC Society: Statement on FDA Warning of Probiotics in Preterm Infants
References
(1) Vallabhaneni S, Walker TA, Lockhart SR, et al. Notes from the field: Fatal gastrointestinal mucormycosis in a premature infant associated with a contaminated dietary supplement–Connecticut, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(6):155-156.
(2) Bizzarro MJ, Peaper DR, Morotti RA, Paci G, Rychalsky M, Boyce JM. Gastrointestinal Zygomycosis in a Preterm Neonate Associated With Contaminated Probiotics. Pediatr Infect Dis J. 2021;40(4):365-367.
(3) Wang Y, Florez ID, Morgan RL, et al. Probiotics, Prebiotics, Lactoferrin, and Combination Products for Prevention of Mortality and Morbidity in Preterm Infants: A Systematic Review and Network Meta-Analysis. JAMA Pediatr. 2023 Oct 2:e233849.
(4) D’Agostin M, Squillaci D, Lazzerini M, et al. Invasive Infections Associated with the Use of Probiotics in Children: A Systematic Review. Children (Basel). 2021 Oct 16;8(10):924.
(5) Rath CP, Athalye-Jape G, Nathan E, et al. Benefits of routine probiotic supplementation in preterm infants. Acta Paediatr. 2023 Jul 28.
(6) Bui A, Johnson E, Epshteyn M, Schumann C, Schwendeman C. Utilization of a High Potency Probiotic Product for Prevention of Necrotizing Enterocolitis in Preterm Infants at a Level IV NICU. The Journal of Pediatric Pharmacology and Therapeutics 2023;28(5):473–475.
(7) Shane AL, Preidis GA. Probiotics in the Neonatal Intensive Care Unit-A Framework for Optimizing Product Standards. JAMA Pediatr. 2023 Sep 1;177(9):879-880.
(8) Su GL, Ko CW, Bercik P, et al. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology. 2020 Aug;159(2):697-705.
(9) WGO Practice Guideline: Probiotics and Prebiotics. Available from: https://www.worldgastroenterology.org/guidelines/probiotics-and-prebiotics
(10) van den Akker CHP, van Goudoever JB, Shamir R, et al. Probiotics and Preterm Infants: A Position Paper by the European Society for Paediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. 2020 May;70(5):664-680.