Probiotics, Prebiotics and Globobiotics!
By Prof. Colin Hill, PhD, APC Microbiome Ireland, University College Cork, Ireland
Growing up I could not imagine what the world would look like in 2020, but I was convinced it would be amazing. The future was exciting, new planets and solar systems would be explored, diseases would be cured, and everyone would have sufficient food and shelter. I sometimes think my generation may have been born at the most perfect time in human history (for someone brought up in a first world country at any rate). We avoided the major world wars which our parents and grandparents endured, we had the benefits of cheap airfares so we could travel the world as tourists, not as armies. Oil was cheap and plentiful. Access to education was widely available. We benefited from antibiotics while they were still effective. Gender inequalities and racism began to be addressed, even though there is still a long way to go. Computers became commonplace and the internet provided access to almost unlimited sources of information.
But here we are in 2020, and now things do not look so promising. Perhaps cynicism is a natural by-product of getting older, but now the future seems to be presented in apocalyptic terms. Climate change, antibiotic resistance, ageing populations, the paradoxes of increasing obesity and increasing hunger, exploding populations, depletion of natural resources and pollution of our oceans. Watching nature programmes hosted by the incomparable David Attenborough has changed from generating a sense of awe at the wonders of the natural world to a sense of despair as to what we are doing to it. Australia is literally on fire as I write this! Can our planet survive the onslaught of the projected 10 billion humans by 2050 – each one hungry for a share of finite resources? Is this really going to be the legacy from my generation to the next – a dystopian future without hope and optimism?
But it’s a New Year and a new decade, and I really want to be hopeful. I am encouraged by the fact that we are gradually beginning to come to grips with this new reality. The UN Sustainable Development Goals provide a roadmap guiding societies and individuals as to how to make a contribution. Attitudes are changing. Too slowly for sure, but we do seem to be at a tipping point.
But what has this tirade have to do with prebiotics and probiotics, you may ask? Well, everything of course. One of the things that really gives me hope is our growing understanding of how humans are simply occupying space in a microbial world. If we squander our opportunity and destroy our planet in terms of human habitation, microbes will carry on for billions of years to come. We should remember that we can only live on Earth because all of the oxygen we breathe is the result of billions of years of microbial metabolism, that most of the carbon cycling on earth is due to microbes, and that every natural system on Earth depends on microbes. Of course we are also inhabited by a vast ecosystem of microbes (our microbiomes) that are required for our health and wellbeing, and we live in environments shaped by microbes. Understanding this will help us to live in harmony with our microbial world, rather than constantly forcing our poor planet to deliver our short term needs.
How can microbes help us to achieve sustainability and restore a healthy ecosystem? I believe that there are many opportunities. By 2050 I predict that we will be using microbes to restore productivity to land damaged by excessive use and pollution. We will be using microbes to clean our oceans of plastic waste. We will improve food production without using chemicals, and we will have certainly reduced food waste (it is estimated that one third of all the food we produce on earth is lost to spoilage, much of it caused by microbes). We will have reduced methane emissions by manipulating the rumen microbiome in domesticated ruminants. We can look forward to a world where we can work with microbes to restore and replenish our atmosphere by unlocking the enormous potential of microbes to scavenge and store carbon. We will have reduced our reliance on antibiotics and will have found microbiome-friendly solutions to prevent and treat infection. We will have developed probiotics and prebiotics that will help us to address metabolic diseases, we will be using bacteriophage to sculpt microbiomes, while psychobiotics will be helping to prevent age related loss of brain function.
Given that the world is a microbial ecosystem, I propose that in the same way we can treat our human ecosystems with prebiotics and probiotics to improve or restore health, we can think in terms of developing microbial solutions to improve or restore planetary health. Because we haven’t had one in at least a month, I propose yet another new term; globobiotics. Globobiotics would be defined as “live microorganisms, microbial products or substrates selectively utilized by microorganisms, that are used in a manner that contributes to the sustainability of our planet”.
We’ve had the Stone Age, the Iron Age, the Oil Age, the Atomic Age and the Information Age, welcome to the Microbial Age!




















 Giving a lecture on beneficial microbes is hard enough to peers sitting in the back of the room, but to do so with young South Africans was more somewhat daunting. However, it proved to be a lot of fun especially when we had to identify kids who were good leaders (the boys all pointed to a girl), who liked to make stuff and sell it to others (two boys stood out). By the end, we had picked the ‘staff’ of a new company.
Giving a lecture on beneficial microbes is hard enough to peers sitting in the back of the room, but to do so with young South Africans was more somewhat daunting. However, it proved to be a lot of fun especially when we had to identify kids who were good leaders (the boys all pointed to a girl), who liked to make stuff and sell it to others (two boys stood out). By the end, we had picked the ‘staff’ of a new company.
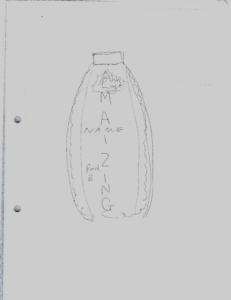 When we re-assembled to present the results, I was impressed with what could be created in such a short time. My favourite was the Amazing Maize, a bottle shaped like a corn cob with the idea it would contain fermented maize. It emphasized the importance of marketing and for products to taste and look good to be purchased.
When we re-assembled to present the results, I was impressed with what could be created in such a short time. My favourite was the Amazing Maize, a bottle shaped like a corn cob with the idea it would contain fermented maize. It emphasized the importance of marketing and for products to taste and look good to be purchased.







