1-10 of 11 results
-

Episode 27: Investigating the benefits of live dietary microbes
[powerpress] The Science, Microbes & Health Podcast This podcast covers emerging topics and challenges in the science of probiotics,… -

Bacterial genes lead researchers to discover a new way that lactic acid bacteria can make energy and thrive in their environments
Lactic acid bacteria are an important group of bacteria associated with the human microbiome. Notably, they are also responsible for… -

ISAPP awards the Glenn Gibson Early Career Research Prize to two diet and gut health researchers
The ISAPP board of directors is pleased to announce that the 2022 Glenn Gibson Early Career Research Prize has been… -
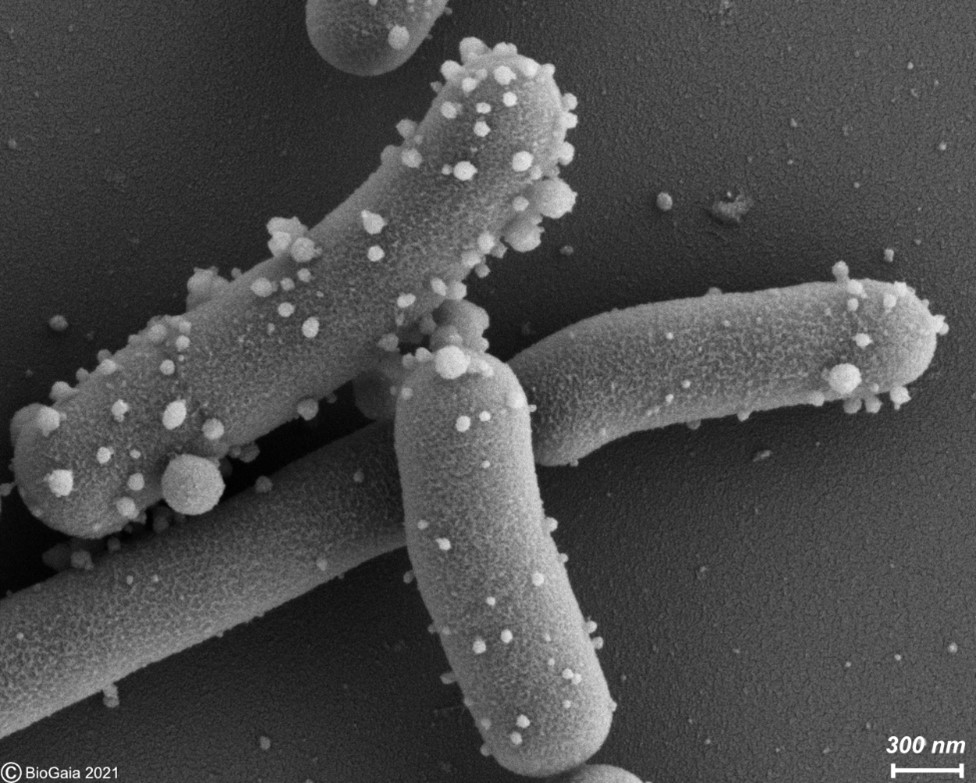
Bacterial vesicles: Emerging potential postbiotics
By Dr. Gabriel Vinderola, PhD, Associate Professor of Microbiology at the Faculty of Chemical Engineering from the National University of… -

ISAPP’s Inaugural Early Career Researcher Prize Awarded to Two Rising Star Probiotic Scientists
This year, ISAPP established a prize for early career researchers with the goal of recognizing individuals who contribute substantial research… -

Connecting with the ISAPP community: Continuing to advance the science of probiotics and prebiotics
By Mary Ellen Sanders PhD, executive science officer, ISAPP On behalf of the ISAPP board of directors, I am reaching… -

ISAPP Students and Fellows Association announce blog posting: A new way to share our work and perspectives
By Anna-Ursula Happel, president ISAPP-SFA and postdoctoral fellow at the Faculty of Health Sciences at the University of Cape Town,… -

The past decade of probiotics and prebiotics research: ISAPP board members share their perspectives.
By ISAPP board members, compiled by Kristina Campbell Scientific progress in the field of probiotics and prebiotics, as in any… -

YOGURITO –the Argentinian social program with a special yogurt
Dra. María Pía Taranto, CERELA-CONICET, Argentina and Prof. Seppo Salminen PhD, University of Turku, Finland It is widely accepted that… -

Role of citizen science in research on fermented foods
By Prof. Sarah Lebeer, Universiteit Antwerpen Spontaneous vegetable fermentations, with their rich flavors and potential health benefits, are regaining popularity…