January 16, 2018. By Mary Ellen Sanders PhD, Sylvie Binda PhD, Seppo Salminen PhD, Karen Scott PhD
Demonstrating health benefits for healthy people is a challenge faced by those attempting to communicate claims on a health promoting food. Foods, in many global regulatory frameworks, are intended for the general population. Therefore, any benefits ascribed to them, the logic goes, must be demonstrated in the generally healthy population.
An old concept has new-found notoriety in the context of offering an approach for establishing health benefits for healthy people. It is the concept of resilience. In an ecological sense, resilience refers to the ability of an ecosystem to withstand perturbation and continue normal function, i.e. maintain homeostasis. In the context of human physiology, resilience enables a host to remain healthy even when exposed to a stress, or to recover from a stress faster. A variety of external challenges such as drugs, pathogens, emotional stress, poor diet among others, may perturb normal physiological function or disrupt the gut ecosystem. Individuals more able to maintain stability of physiological functions when exposed to such challenges would be healthier than those who cannot maintain stability. Thus, a food would be considered to have a beneficial effect if it could increase the resilience of the consumer to a challenge.
This concept was described in an EFSA guidance document on biological relevance of data in scientific assessments:
“When subject to a disturbance, a biological system enters in a transient state: a process variable has been changed and the system has not yet reached steady state. Some systems, including humans, have the capacity to regulate their internal environment and to maintain a stable, relatively constant condition of properties; it is called ‘homeostatic capacity’. Resilience represents the amount of disturbance that can be absorbed by a system before the system changes or loses its normal function, or the time taken to return to a stable state, within the normal operation range following the disturbance…” [Reducing] “homeostatic capacity … might be detrimental, whereas increasing the capacity could be beneficial.”
This concept aligns with the definition of ‘health’, which includes the ability to adapt to the environment.
Resilience of gut microbiota
This concept of resilience can be applied to the human microbiota as an ecosystem. Once established in early childhood, our colonizing microbiota reaches a relatively stable state. Although brief fluctuations occur, especially in relation to daily diet and medicines used, the microbial ecosystem of a healthy adult provides relatively stable functionality. Disruption of the microbiota by repeated stressors can be associated with poorer health. There seems to be a solid rationale that the ability of the colonizing microbiota to resist, or recover quickly from, perturbations reflects a person’s ability to remain healthy. The microbiota stability may be indicated in either populations of bacteria or their metabolic output.
Homeostasis and health: a statistical approach
“A statistical approach to measuring improved health was proposed by Dr. Dan Tancredi at the 2010 ISAPP meeting. It is reprinted here from: Sanders, et al. 2011. Health claims substantiation for probiotic and prebiotic products. Gut Microbes 2:3, 1-7.
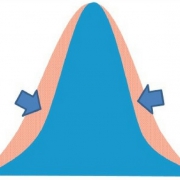
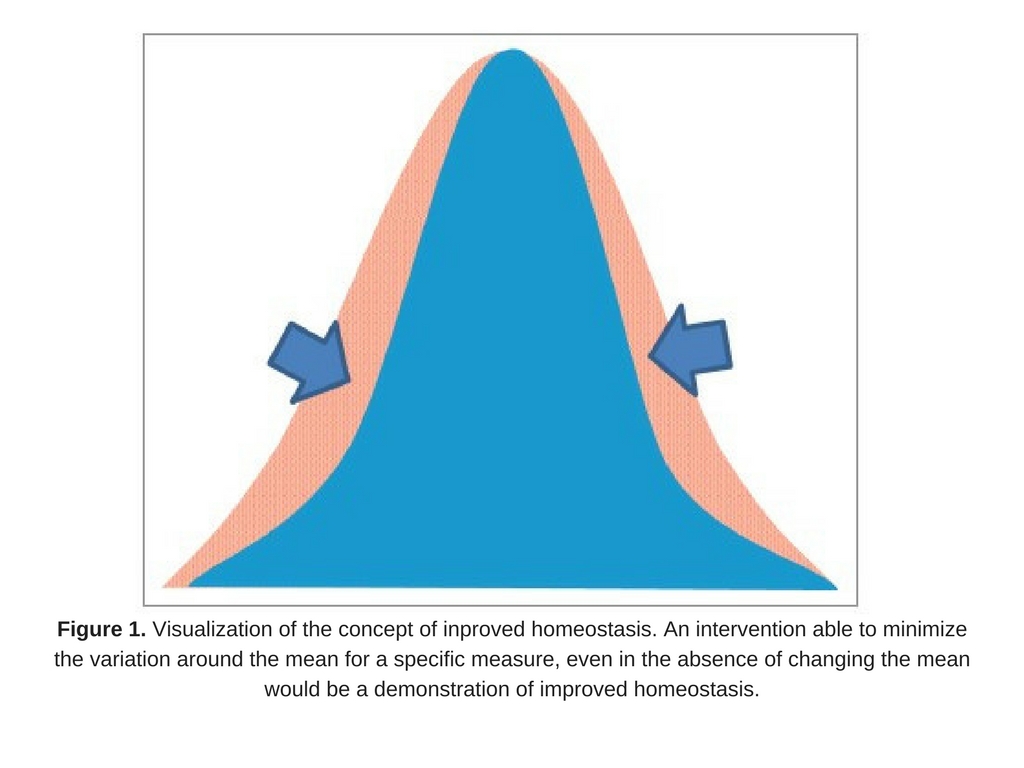
An approach to measuring improved health may be to measure homeostasis, as suggested by D. Tancredi. From a statistical point of view, if an intervention were able to minimize the variation around the mean for a specific measure (even in the absence of changing the mean; Fig. 1), it could be a reflection of improved health, assuming a biological rationale exists that tighter control of the parameter is physiologically advantageous. In other words, lessening the fluctuation around an individual’s biomarker could be interpreted as contributing to improving health. This novel idea emphasizes the importance of homeostasis as a focus of studies on health, and provides a rationale based in solid statistical theory as a way to measure this.
One challenge to demonstrating the value of this approach is to identify appropriate biomarkers that could be studied. The following properties would be important to a relevant biomarker for homeostasis:
- maintaining moderate levels of the biomarker is associated with good health;
- high or low values are associated with ill health;
- biomarker levels in the same person can fluctuate over time; and
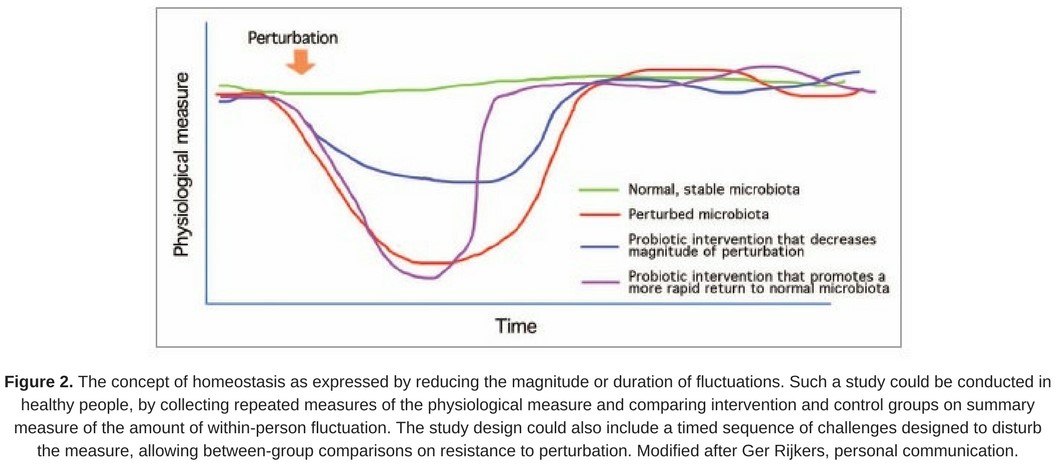
- reducing the magnitude or duration of such fluctuations in healthy people is considered desirable (Fig. 2).
Such a biomarker could be an individual endpoint or be formed as a ratio of two other biomarkers, when maintaining the same relative amounts of the two component biomarkers would be desirable.
Assuming a biomarker with the above properties is available, it could be used as the outcome measure in a randomized controlled trial to provide evidence that the experimental food is able to improve the maintenance of health in humans. Statistically, the trial would be set up to address the hypothesis that the experimental substance is associated with lower variation in biomarker levels, compared to the control arm, in subjects who were healthy at baseline. Such a trial would be able to use information on within-person variations in biomarker levels, even those who did not become ill. Partly as a result of the more efficient use of study data, such a trial would require far fewer subjects than an intervention that instead addressed the hypothesis that treatment is associated with fewer healthy persons becoming ill.
A mounting understanding of the value of stability of the colonizing microbial communities makes this endpoint an attractive one to consider. Perturbation of gut microbiota is associated with intestinal dysfunction, as illustrated during antibiotic treatment. Specific probiotics have been shown to promote a quicker rebound from antibiotic-induced microbiota disruption, including a study on Lactobacillus rhamnosus GG (LGG) (Cox et al. 2000). This paper concludes ‘…that a key mechanism for the protective effect of LGG supplementation on the subsequent development of allergic disease is through the promotion of a stable, even and functionally redundant infant gastrointestinal community.’
However, it would be useful to define additional biomarkers that would be appropriate targets for this type of investigation.
In pediatric nutrition, the measurement of metabolic homeostasis has become a standard approach when developing infant formulas (Heird, 2005). The concept of homeostasis as a model to distinguish between foods (including food supplements) and medicinal products was explored by the Council of Europe (2011), and is an interesting correlate to the above hypothesis.”
Conclusions
The recent recognition by EFSA that maintenance of homeostasis is a valid measure of health provides an opportunity to apply this concept to validate health benefits of specific foods and food ingredients. Stability of microbial populations, microbial metabolism or host physiological readouts could be measured to reflect the concept of resilience. Since there is no definitive composition of a ‘healthy human microbiota’, a more reasonable target for measuring positive impacts of a probiotic on the microbiota would be reflected not in absolute levels of specific microbes but in the ability of a specific probiotic or prebiotic to bolster the resilience of the microbiota.
References:
Council of Europe. Homeostasis, a model to distinguish between foods (including nutritional supplements) and medicinal products 2008; (Accessed February 24, 2011, at http://www.coe.int/t/e/social_cohesion/soc-sp/homeostasis%20%282%29.pdf ).
Cox MJ, Huang YJ, Fujimura KE, Liu JT, McKean M, Boushey HA, et al. Lactobacillus casei abundance is associated with profound shifts in theGunderson LH, 2000. Ecological resilience: in theory and application. Annual Review of Ecology and Systematics, 31, 425–439.
EFSA guidance document: Guidance on the assessment of the biological relevance of data in scientific assessments; July 12, 2017; EFSA Journal 2017;15(8):4970
Heird WC. Biochemical homeostasis and body growth are reliable end points in clinical nutrition trials. Proceedings of the Nutrition Society 2005; 64:297-303.
Huber M, Knottnerus JA, Green L, van der Horst H, Jadad AR, Kromhout D, Leonard B, Lorig K, Loureiro MI, van der Meer JW, Schnabel P, Smith R, van Weel C, Smid H (2011). “How should we define health?” BMJ. 343:d4163.
Sanders, et al. 2011. Health claims substantiation for probiotic and prebiotic products. Gut Microbes 2:3, 1-7; May/June 2011