What do we mean by ‘conferring a health benefit on the host’?
By Prof. Colin Hill, University College Cork, Ireland
Four of the Consensus definitions produced by ISAPP in recent years (see 1-4 below) finish with a similar wording, insisting that probiotics, prebiotics, synbiotics and postbiotics must “confer a health benefit on the host”. This proviso was included to explicitly reinforce the fact that the raison d’etre for these interventions is that they must demonstrably improve host health. It would perhaps be wise to just stop there and leave the interpretation of what this really means to each individual reader. But that would not make for a very long blog and I am not very wise. Furthermore, it is useful to be more precise for scientific and regulatory purposes. At least two aspects seem to be open to elaboration; what is meant by ‘host’ and what is a ‘health benefit’? I will base my thoughts on the probiotic definition, but the logic should apply equally to all four health-based definitions.
Host. According to the Google dictionary a host is ‘an animal or plant on or in which a parasite or commensal organism lives’. This means there are millions of potential host species on our planet, something that could potentially create confusion. For example, if a well characterised microbe (or microbes) is shown to provide a measurable health benefit when administered in adequate amounts in a murine model (the host) then it clearly meets the stated definition of probiotic. But only for mice! It should not be referred to as a probiotic for other species, including humans, solely based on murine evidence. This creates a situation where the same microbe can clearly meet the criteria to be a probiotic for one host but not for another. This is not simply semantics; it is of vital importance that it should not be assumed that health benefits confirmed in one host will also be realised in another without supporting evidence. Since the majority of discussions of probiotics address human applications, it may serve all stakeholders well – even if not directly mandated by the definition – if the word ‘probiotic’ was only used without qualification for microbes with measurable benefits in humans while all others should be qualified with the target host; ‘equine probiotic’, ‘canine probiotic’, or even ‘plant probiotic’.
Health benefit. Health is of course a continuum from a desirable but almost certainly unattainable state where every organ is performing optimally (something I will term ‘ideal health’) to a point where death is imminent (that I will term ‘poor health’). Of course, health is multidimensional and far more complex than a straight line between ‘ideal’ and ‘poor’ but for simplicity I will treat it as such. If we place ideal health on the left end of our straight line and poor health at the right end, then obviously any shift towards the left can be considered a health benefit. It could even be reasonably argued that if someone is gradually progressing from left to right down our imaginary line (for example, as we age) then halting or slowing down that progression could also be considered a health benefit. From this perspective every individual (not just the unwell) could potentially derive a health benefit from a probiotic, prebiotic, synbiotic or postbiotic.
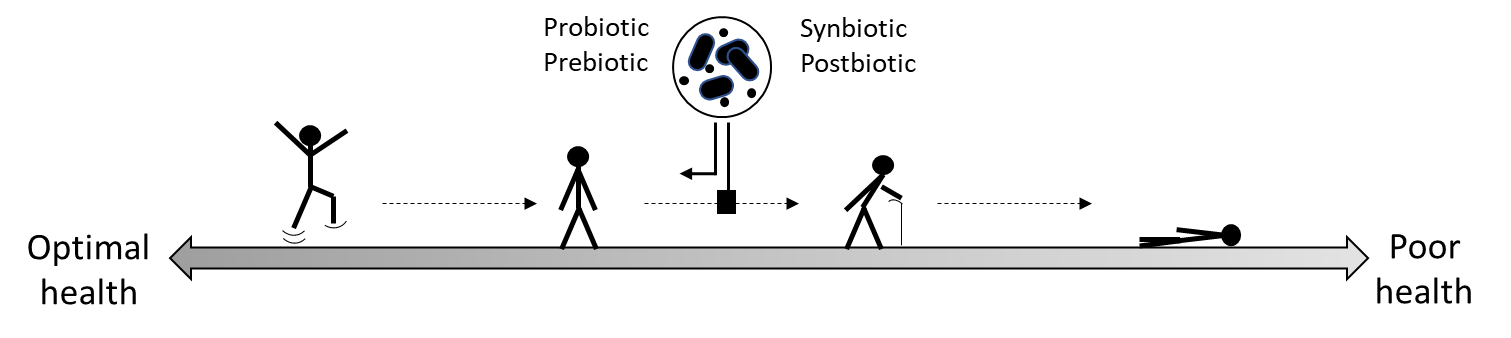 The issue of cosmetic benefits is more nuanced. If an intervention improves someone’s appearance (or reduces body odour for example) it might not be considered a health benefit per se, but of course it could well have a beneficial effect on an individuals’ mental health. I will leave it to the psychologists and psychiatrists to determine how this could be convincingly demonstrated.
The issue of cosmetic benefits is more nuanced. If an intervention improves someone’s appearance (or reduces body odour for example) it might not be considered a health benefit per se, but of course it could well have a beneficial effect on an individuals’ mental health. I will leave it to the psychologists and psychiatrists to determine how this could be convincingly demonstrated.
There is also the issue of production characteristics where the host is a food animal or a crop. If a microbial-based intervention leads to faster growth rates and increased yields should this qualify as a health benefit? My own opinion is if the intervention leads to higher productivity by preventing infections it could be considered a health benefit, but not if it simply leads to faster growth rates by improving feed conversion for example.
Can changing the microbiome be considered a health benefit? A trickier question is whether a direct effect on the microbiome could be considered as a health benefit? Every host has a microbiome of a particular configuration, richness, and diversity. I don’t think we are yet at a point where measurable changes in these general indices of microbiome composition can be termed a health benefit in the absence of a link to a more established health outcome. The consequence of any change will be microbiome-specific in any event; a reduction in diversity in the vaginal microbiome might be desirable, whereas an increase in diversity in the gut microbiome might well be considered beneficial. But what if we can measure a reproducible reduction in a specific pathobiont like Clostridioides difficile, or an increase in a microbe that is associated with good health such as Bifidobacterium? In my opinion we are arriving at a point where we can begin to refer to these impacts as a health benefit. This will become more and more relevant as we establish direct causal links between individual commensal microbes and health outcomes. Equally, an intervention that preserves microbiome structure during a disruption (e.g. infection or antibiotic treatment) could also be considered as beneficial. I don’t know if regulators are yet at the point of accepting outcomes such as these as direct health benefits, but I believe a strong case can be made.
To finish, I believe that it is a very exciting time for all of us in the field of probiotics, prebiotics, synbiotics and postbiotics, but it is really important that all of this important science is not compromised by loose language or by literal interpretations that adhere to the letter of the definitions but not to the intent. If you want to fully understand the intent of the definitions, I encourage you to read the full text of the consensus papers.







