1-10 of 10 results
-

Probiotics vs. prebiotics: Which to choose? And when?
As consumers, we are constantly bombarded with information on what we should eat to improve our health. Yet the information… -

Do antibiotics ‘wipe out’ your gut bacteria?
By Dr. Karen Scott, University of Aberdeen, UK Antibiotics have been an important tool in medicine to kill pathogenic bacteria… -

Probiotics: Money Well-Spent For Some Indications
Eamonn M M Quigley MD, Houston Methodist Hospital and Weill Cornell Medical College, Houston, Texas, USA; Hania Szajewska MD, The… -

Late initiation of probiotic therapy for acute pediatric gastroenteritis may account for null results
Francisco Guarner, MD, PhD, University Hospital Vall d’Hebron, Barcelona, Spain; Michael Cabana, MD, MPH, University of California, San Francisco, CA,… -

ISAPP-initiated systematic review and meta-analysis shows the association of probiotic consumption with reduced antibiotic prescriptions
At the ISAPP meeting in Turku, Finland in 2016, scientists convened a working group led by Dan Merenstein of Georgetown… -

Clinical evidence and not microbiota outcomes drive value of probiotics
By ISAPP Board of Directors, plus Prof. Francisco Guarner and Dr. Bruno Pot September 10, 2018 Two recent papers have… -
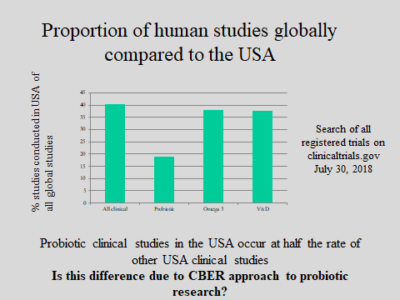
CBER to hold public workshop on regulation of biologics
FDA’s Center for Biologics Evaluation and Research (CBER) is convening a public workshop Sept 17 in Rockville MD on the… -

ISAPP Goes to India
By Mary Ellen Sanders PhD and Dan Merenstein MD ISAPP sent two key-note speakers to the Probiotics Association of India… -
ISAPP works to have evidence-based usage of probiotics to prevent antibiotic-associated diarrheoa implemented in UK
January 12, 2018. Antibiotics are amongst the most commonly prescribed drugs in UK hospitals. However, as well as treating infection… -
The Times They Are A-Changin’ With Probiotics
I had a surprising encounter a few weeks ago in the clinic. I was caught off guard, had to take…