Can dietary supplements be used safely and reliably in vulnerable populations?
By Dr. Greg Leyer, Sr. Director – Scientific Affairs, Chr. Hansen, Inc., Madison, WI and Prof. Dan Merenstein, Department of Family Medicine, Georgetown University Medical Center, Washington DC
What is it that doctors look for when recommending or prescribing therapies to patients? If it is a drug, a supplement, a new diet, or even a new exercise regimen, they look for safety and efficacy. There are of course other things to consider, including cost, ease of administration, and patient compliance, among others. But safety and efficacy are their foremost concerns.
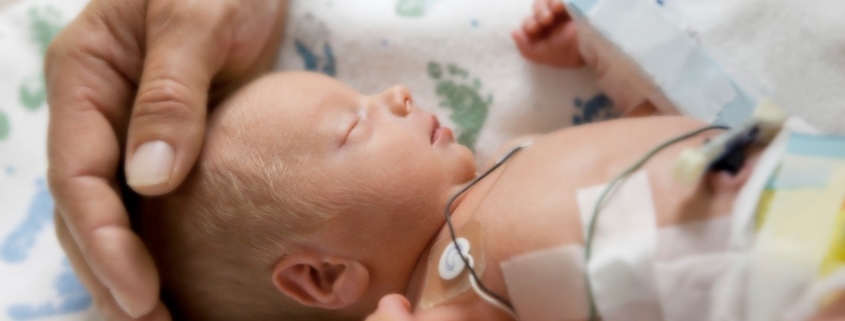
A recently published clinical report from the American Academy of Pediatrics (AAP) (Poindexter 2021) examined the evidence for probiotics to prevent morbidity and mortality in preterm infants. They concluded that probiotics could not be recommended. This differs from conclusions of the American Gastroenterological Association (AGA) (Su et al. 2020), which recommended specific probiotic strains for preterm (less than 37 weeks gestational age) and low birth weight infants. The AAP report also differs from the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) (Van den Akker et al. 2020), which recommends specific strains for this use, although their recommendations are not fully aligned with AGA’s (see What’s a Clinician to do When the Probiotic Recommendations from Medical Organizations Do Not Agree?).
The AAP report does a thorough job of reviewing data on use of probiotics in the NICU, including conflicting studies, lack of confirmatory studies of efficacious strains, and safety and cross contamination inside the NICU. However, the overriding theme of the report is “clinicians must be aware of the lack of regulatory standards for commercially available probiotic preparations manufactured as dietary supplements and the potential for contamination with pathogenic species.” Therefore, at the heart of the AAP failure to recommend probiotics is the concern that the quality of available products is insufficient. Because of the absence of a pharmaceutical-grade probiotic product for use in the United States, they posit, they cannot recommend usage. It is noteworthy that the trials performed on premature infants resulting in multiple conclusions of safety and efficacy have thus far utilized probiotic products manufactured as dietary supplements.
Probiotics can be marketed as drugs if they follow that regulatory pathway, but generally in the US they are sold under the regulatory classification of dietary supplements. Is the AAP correct that no dietary supplement is of sufficient quality to recommend for use in preterm infants?
Quality of probiotic dietary supplement probiotics. Dietary supplements were a category of product developed to supplement the diet of the generally healthy population, not to treat or prevent disease. In practice this is an important distinction, because while the safety standard is high for dietary supplements for healthy individuals (see comments by food safety expert Jim Heimbach here), such supplements do not need to be established as safe for patient populations. But in the case of probiotics, many clinical trials have evaluated safety and efficacy for prevention or treatment of disease, more aligned with drug uses. Yet probiotic products supported by these data are not marketed in the US as drugs.
It is a common misperception that dietary supplements are “not regulated”. However, the FDA has clear good manufacturing practices (GMP’s) and regulations dedicated to dietary supplement manufacturing. The onus is on manufacturers to establish appropriate product specifications based on intended use and risk. Reputable manufactures establish rigorous purity, strength, and identity quality standards consistent with the intended population and sufficient for that use. Products intended for infants, including premature infants, should be manufactured under quality standards more rigorous than those intended for a healthy adult population. For example, Chr. Hansen bases the enhanced specifications for products aimed at infants, and preterm infants, on elements of Codex standards for infant formula, amongst other stringent microbiological criteria. This would include manufacturing the probiotic strain to an “infant” grade, employing stricter environmental monitoring, sanitation, and airflow control throughout the process, careful selection of raw ingredients for infant compatibility, and enhanced testing and purity standards using validated methods at every step. The internal manufacturing standards that Chr. Hansen applies for products intended for infants, and preterm infants, are much stricter than typical dietary supplement standards, and are appropriate for their intended use.
Therefore, there are high quality, safe probiotic products produced under dietary supplement regulations even though such products do not carry any label statement claiming this added level of quality. However, products sourced for hospitals to stock in formularies could work with the supplier to demand this extra level of product testing specifications. Pharmacies can institute quality agreements with vendors that would delineate their expectations for the strains present, the levels of live microbes acceptable in the final product, etc. This agreement could also mandate that any product change – as defined in the agreement – would require the vendor to notify the customer. Such an agreement might be burdensome for a hospital pharmacist, but a sophisticated dietary supplement company should be able to assure the hospital formulary of their quality.
Products made using strict specifications, geared towards infant and premature infant applications, are on the market and are safely being used in this patient population in many NICUs and as part of infant formulas. We disagree with AAP’s position that a pharmaceutical approach is needed, as long as a product of sufficient quality can be provided. To deny preterm infants probiotics, which have a significant chance of improving their clinical outcomes, is not in line with other medical recommendations. Instead, the hospital formularies should stock products that have been scrutinized for sufficient evidence of safety and efficacy. Suppliers of stocked products should provide product testing results, a description of the quality standards employed during production, and a rationale for the suitability of the standards for preterm infants. Third party verification of adherence to these quality standards would assure medical professionals regarding the safety of these products for use.
References
CAC/RCP 66-2008. Code of hygienic practice for powdered formulae for infants and young children. Codex.
Poindexter, B. 2021. Use of Probiotics in Preterm Infants. Pediatrics 147 (6): e202 1051485.
Su et al. 2020. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology 159:697-705.
Van den Akker et al. 2020. Probiotics and Preterm Infants: A Position Paper by the European Society for Paediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition Working Group for Probiotics and Prebiotics. Journal of Pediatric Gastroenterology and Nutrition. 70(5):664-680.