Should the concept of postbiotics make us see probiotics from a new perspective?
By Dr. Gabriel Vinderola, PhD, Associate Professor of Microbiology at the Faculty of Chemical Engineering from the National University of Litoral and Principal Researcher from CONICET at Dairy Products Institute (CONICET-UNL), Santa Fe, Argentina
In early May 2021 an ISAPP consensus panel defined postbiotics as “a preparation of inanimate microorganisms and/or their components that confers a health benefit on the host“. The fact that non-viable microbes may still deliver health benefits is not new for the scientific community and was reviewed more than 20 years ago. More recent studies demonstrating health effects of non-viable microbes spurred interest in this topic, leading ISAPP to carefully consider the emerging use of the term ‘postbiotic’ and provide a clear, modern, concise definition.
Postbiotics can be contrasted with probiotics: live microorganisms that, when administered in adequate amounts, confer a health benefit on the host. In practice, probiotics have likely always coexisted with inanimate microbes, as live microbes will die at all phases of production of a product. In the past, it seems the presence of inanimate microbes as part of probiotic products was not really considered. We all knew they were there, but made a default assumption that they had limited significance. As we consider postbiotics, though, we should perhaps look again at how to address the inanimate components of probiotic products.
The presence of inanimate cells in probiotic preparations: from lab to product
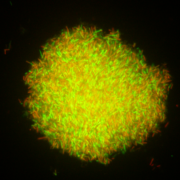
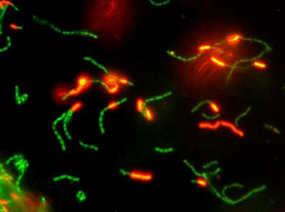
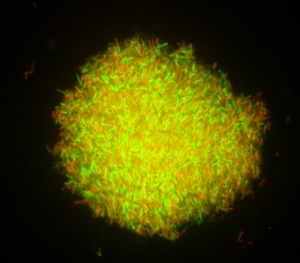
A loop of a fresh, overnight, live culture of a probiotic strain may still contain non-viable cells (Fig. 1). During the biomass production of a probiotic culture, an abundance of live cells can be observed in the exponential growth phase, but as the culture enters the stationary phase, a significant increase in the proportion of non-viable cells occurs (Fig. 2). Yet the culture may display a satisfactory high number of viable cells as verified by traditional plating on agar media. Some years ago, it was reported that a fresh culture of a lactobacilli strain may display a live/dead cells ratio of ca. 100/1. However, this ratio may change to 1:1 after freeze-drying, as studied using flow cytometry, a technology that allows the quantification of both live and dead cells in a culture-independent way. Therefore, a recently freeze-dried culture of a probiotic strain may contain 1010 log CFU/g of live cells, but also the same amount of non-viable cells.
Food supplements may have a shelf life between 12 and 24 months at room temperature and over this time, a proportion of cells will likely lose viability along the shelf life. This depends on the intrinsic resistance of the strain, the nature of the matrix used for freeze-drying, the water activity remaining after lyophilization, the package and the storage conditions. Taking this into consideration, the probiotic supplements industry overfills probiotic capsules or sachets with 1.5 to 4 times more live cells, in order to warrant the delivery at the end of shelf life of the minimum amount of live cells to be able to deliver the expected health benefit. Considering that both freeze-drying and long-term storage may significantly increase the proportion of inanimate microorganisms in a probiotic supplement, a probiotic supplement could easily consist of more inanimate microorganisms than live ones. Yet if the products delivers the minimum amount of live cells to confer a health benefit, this makes the product fit the definition of probiotics so it must be considered a probiotic product. The probiotic focus has been prevalent during previous clinical trials and also during the shelf life of a probiotic product. Maybe we were just overlooking what was going on beyond the information obtained by CFU. These new insights do not change the status of a probiotic, but with due attention given to postbiotic components, offers the possibility to have better and better characterized products in the future.
Figure 1, above – Fluorescence microscopy images of an overnight (18h) culture of bifidobacteria (left) and lactobacilli (right) showing live (green) and non-viable cells (red). The Live/dead BackLight Invitrogen® kit was used for staining cells.
Figure 2, above – Fluorescence microscopy images of a culture of lactobacilli in the exponential (left) and late stationary (right) growth phase showing live (green) and non-viable cells (red). The Live/dead BackLight Invitrogen® kit was used for staining cells.
Are dead probiotics ‘postbiotics’?
What is the contribution of these inanimate cells to the overall health benefit observed for the probiotic culture? In most cases, no evidence exists documenting health benefits of inanimate probiotics. But we may have reason to suspect it may be relevant. For example, a live culture of Bifidobacterium bifidum MIMBb75 significantly alleviated irritable bowel syndrome symptoms and improved quality of life in a double-blind, placebo-controlled study when delivered at 109 CFU, but also the same strain performed equally well for the same end-point when delivered as a heat-inactivated culture. Also, a novel next-generation probiotic strain of Akkermansia muciniphila performed equally well in its live and pasteurized form for improving several metabolic parameters in overweight and obese volunteers. In these cases, it can be said that both strains fit simultaneously the probiotic and the postbiotic definitions.
However, does this mean that as the strain gradually loses viability during storage it gradually becomes a postbiotic? No! This is because method of strain inactivation may play an important role in the health benefit observed. For example, the health benefit delivered by a strain that underwent a heat inactivation can not be assumed to have the same functionality if it is left to die on its own on the shelves. A heat treatment may, for instance, modifiy the spatial display of surface proteins and this may lead to a different immunomodulating capacity of the strain when compared to spontaneous and gradual cell viability lost along storage.
Characterizing probiotic products with an eye to the presence of non-viable cells
By definition, probiotics must be quantified. In the past, this quantification has been limited to numbers of viable cells, typically using a colony count method. This is wholly appropriate, as probiotics must be alive. Yet for the future, will it become necessary to quantify the numbers of non-viable microbial cells as well? With evidence emerging that these non-viable cells may be functional components, then a reasonable argument can be made that this component of a probiotic product should also be quantified. This has implications for characterizing products for use in intervention trials and for the marketplace. The challenge for the marketplace is that probiotic products should deliver the functionality observed in intervention trials.
Reports of trials typically indicate a viable count of the probiotic being tested, but these can be reported in different ways. For example, the statement may indicate delivery of 1.9 × 107 CFU/day of the strain XXX, or delivery of > 1.9 × 107 CFU/day of the strain XXX. These are very different and neither gives any indication of the level of non-viable microbes. The first expression is a specific measure of the viable count at a particular point in time. The second indicates a target minimum and the actual count of viable cells could be much greater. Counts all along the intervention are rarely reported, even though that count could change substantively over time. Papers rarely report if the same batch or different batches of the probiotic preparation were used. The potentially increasing proportion of inanimate microbes is never reported.
In light of postbiotics, future studies should report quantifications of both live and inanimate microbes. Although it is not clear at this time what role inanimate microbes may play in probiotic efficacy, a first step is understanding the composition of probiotic products.
ISAPP members can access Dr. Vinderola’s webinar on this topic here. Email info@nullisappscience.org if you require the password.