Probiotics to Prevent Necrotizing Enterocolitis: Moving to Evidence-Based Use
By Ravi Mangal Patel, MD, Msc, Associate Professor of Pediatrics, Emory University School of Medicine and Children’s Healthcare of Atlanta. rmpatel@nullemory.edu Twitter: @ravimpatelmd
Necrotizing enterocolitis (NEC) is one of the most lethal neonatal diseases, yet most people have never heard about it. The disease primarily affects preterm infants and is characterized by the development of intestinal inflammation. Clinically, the disease often manifests with an infant developing feeding intolerance or abnormal abdominal exam findings. The diagnosis is confirmed by abdominal x-ray or ultrasound. One of the key diagnostic radiographic findings is pneumatosis, which is air in the lumen of the bowel caused by gas-producing bacteria.

Dr Ravi Mangal Patel
NEC accounts for 1 out of every 10 deaths in US neonatal intensive care units. Among extremely preterm infants (those born at 22-28 weeks’ gestation) in the US, NEC is the most common single cause of death between 2 weeks and 2 months of age. Many infants with NEC undergo surgery to remove diseased bowel and those who recover and survive are at risk for long-term neurodevelopmental impairment and short bowel syndrome.
Decades of research into NEC have identified several key risk factors, including formula feeding, inconsistent feeding, abnormal intestinal oxygenation and [gut microbiota] dysbiosis. Studies have shown that dysbiosis, or abnormal intestinal colonization, is an important antecedent risk factor for the development of NEC. These studies have found that infants who develop NEC have an increase or bloom in the relative abundance of proteobacteria, compared to those who do not develop NEC. These proteobacteria, which contain a lipopolysaccharide coating, may lead to inflammation through their interaction with Toll-like receptor 4.
Given the role of dysbiosis in NEC, efforts to intervene by provision of probiotics to prevent NEC is a rational and extensively studied intervention, with over 63 randomized trials enrolling ~15,000 infants to date. The aforementioned meta-analysis, along with several others (Table 1), show probiotic supplementation results in large magnitude reductions in the risks of NEC and death and more modest reductions in the risks of late-onset sepsis. However, there is more limited data on extremely preterm infants and the quality or certainty of evidence for probiotics for the prevention of NEC was low in a recent Cochrane review.
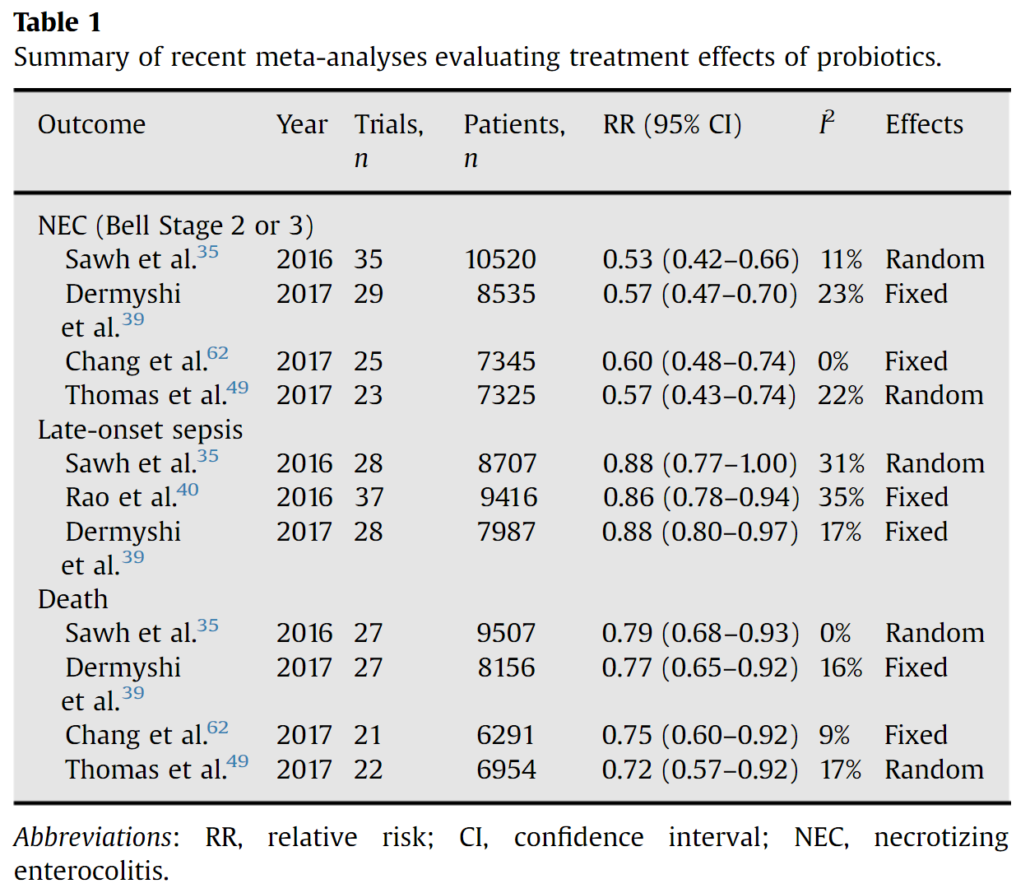
Source: https://doi.org/10.1053/j.sempedsurg.2017.11.008
In the United States, an increasing number of centers have begun to routinely provide probiotics, with the greatest increase in use beginning in 2015. Observational studies evaluating routine probiotic use show benefits that are similar in magnitude to those from randomized trials, supporting the external validity of the results from the trials. This includes a large recent evaluation of probiotic use in the United States. Around the world, probiotic use is highly variable, from 100% of NICUs in New Zealand, 68% of NICUs in Germany, to 12% in the UK, 21% in Canada and 14% in the United States. Some of the variability in clinical use may be related to the uncertainty regarding the quality of commercially available probiotic products and need for clarity regarding strain-specificity of effects. There are many considerations both for and against routine use of probiotics to prevent NEC (Table 2). Current probiotic dietary supplements do not undergo FDA’s premarket review and approval requirements for safety and effectiveness or have to meet manufacturing and testing standards for drugs, and the potential risks were highlighted by a case of an infant death from a contaminated supplement. There is currently no FDA-approved live biotherapeutic product to prevent NEC.
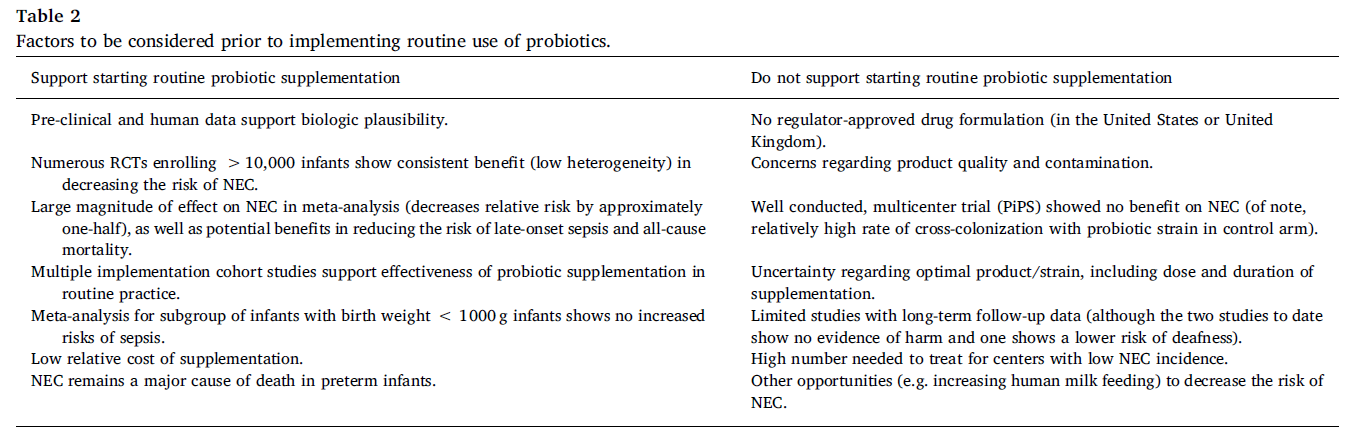
Source: doi: 10.1016/j.earlhumdev.2019.05.009
Recent recommendations and guidance from ESPHGAN and the AGA also demonstrate that some medical organizations recognize the strength of the data in support of probiotic use to prevent NEC. It has been over two decades since the first study demonstrating the benefit of probiotic supplementation to prevent NEC in preterm infants. Now, more than ever, the evidence continues to accumulate regarding the beneficial effects of probiotic use in preterm infants as a compelling strategy to reduce the risks of both NEC and death. Therefore, considering the balance of potential risks and benefits including data from both randomized trials and routine implementation studies, my opinion is that the cumulative evidence to date supports routine probiotic use to prevent NEC and death in preterm infants.
As important is considering the parent voice regarding probiotic use. The NEC Society is a non-profit focused on NEC that has worked to incorporate the voice of the patient-family in clinical decisions.
Disclosures: Dr. Patel serves on the data-safety monitoring board of the Connection Study, which is a trial examining the use of an investigational probiotic to decrease the risk of NEC.
For further information, see this seminar by Dr. Patel: Practical Consideration for Probiotics in the NICU






