Episode 18: The definition of postbiotics

Podcast: Play in new window | Download
Subscribe: Apple Podcasts | Spotify | RSS
The Science, Microbes & Health Podcast
This podcast covers emerging topics and challenges in the science of probiotics, prebiotics, synbiotics, postbiotics and fermented foods. This is the podcast of The International Scientific Association for Probiotics and Prebiotics (ISAPP), a nonprofit scientific organization dedicated to advancing the science of these fields.
The definition of postbiotics, with Dr. Gabriel Vinderola and Prof. Seppo Salminen
Episode summary:
In this episode, the ISAPP podcast hosts join guests Gabriel Vinderola, PhD, Principal Researcher at the
National Scientific and Technical Research Council (CONICET) and Associate Professor at University of Litoral in Argentina, and Seppo Salminen, PhD, Professor at University of Turku in Finland, to discuss the relatively recent definition of postbiotics and what kinds of substances are included in this category. They talk about the criteria for something to qualify as a postbiotic, common mechanisms of action for postbiotics, and how postbiotic science has brought new perspectives on the study of probiotics.
Key topics from this episode:
- What are postbiotics? Dr. Vinderola and Prof. Salminen dive deep into the definition of postbiotics created in 2021 and what it entails.
- Postbiotics, similar to probiotics, prebiotics, and synbiotics, must provide health benefits to the host.
- The nature of the postbiotic preparation is important for its health benefits. When the inactivation process is changed, this can lead to altered health benefits, and clinical studies must be repeated to ensure the desired health benefits are maintained.
- They explain why “inanimate” was chosen to describe the microorganisms / components in a postbiotic preparation.
- What is the mode of action, or how do postbiotics work?
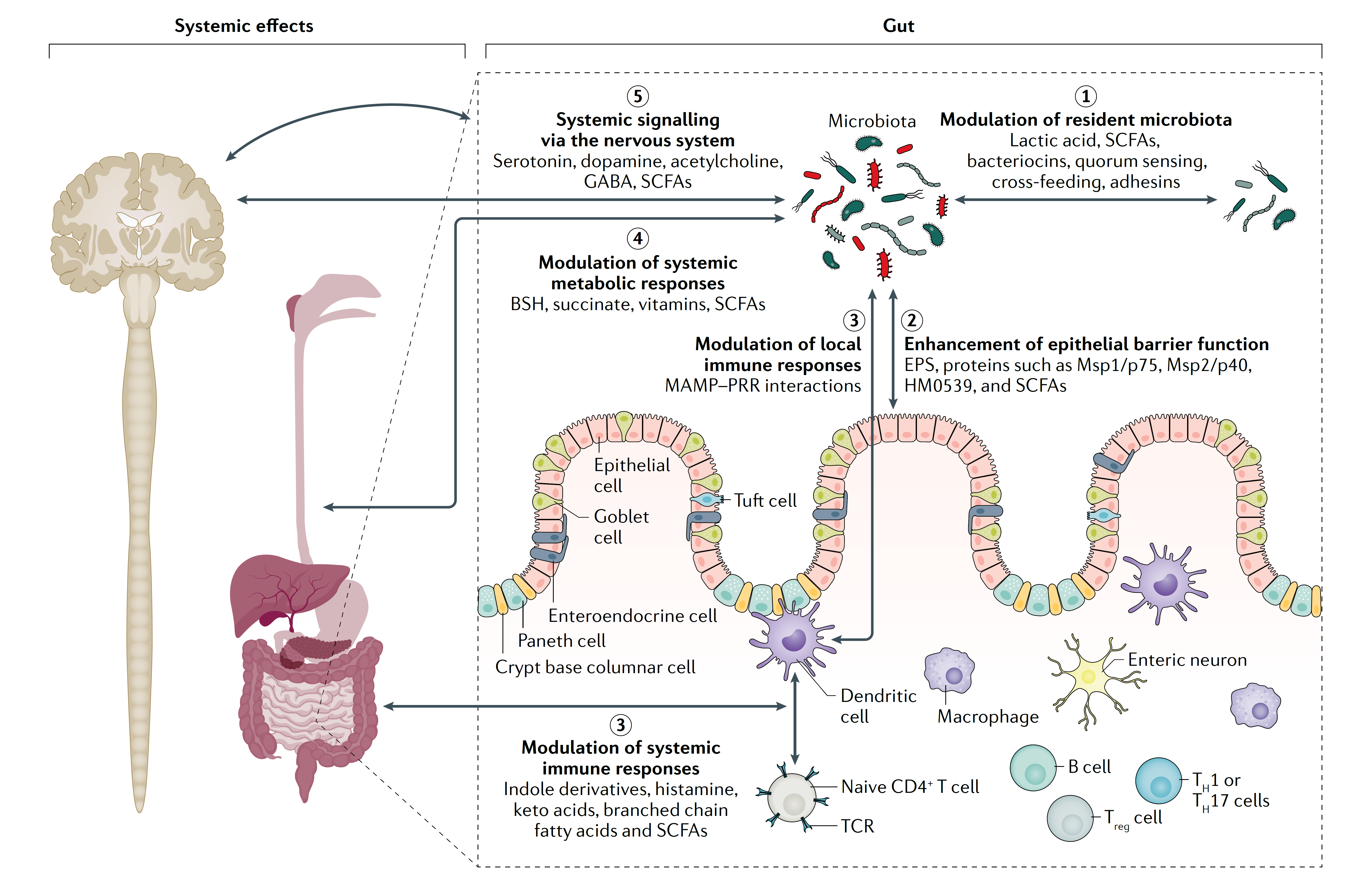
- Postbiotics show similar mechanisms of action to probiotics, except for ones requiring viability, since postbiotics will not grow and produce metabolic byproducts in the host.
- Postbiotics can benefit the host via physical interaction with the host epithelial and immune cells.
- A primary mechanism of action is likely to be through activation of the immune system, through which postbiotics can affect inflammation and some disease conditions.
- Postbiotics may also affect the microbiome composition and ability to inhibit pathogens.
- From a regulatory point of view, inanimate microorganisms may represent an easier category to prove safe for users. For industry, postbiotics may be more convenient with a longer shelf life.
- Some controversy still exists around the ISAPP-led postbiotic definition, and this has led to valuable discussions that are crucial to scientific progress. So far the authors of the definition have defended their stance.
Episode abbreviations and links:
- ISAPP scientific consensus definition of postbiotics, published in Nature Reviews Gastroenterology & Hepatology: The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics
- Study on a postbiotic for addressing chronic stress in students: Health Benefits of Lactobacillus gasseri CP2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double-Blind, Placebo-Controlled Study
- Study using live bifidobacteria to address irritable bowel syndrome: Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–a double-blind, placebo-controlled study
- Study using dead bifidobacteria (same strain as above) to address irritable bowel syndrome: Heat-inactivated Bifidobacterium bifidum MIMBb75 (SYN-HI-001) in the treatment of irritable bowel syndrome: a multicentre, randomised, double-blind, placebo-controlled clinical trial
- Opinion from the European Food Safety Authority (EFSA) Panel on Nutrition, Novel Foods and Food Allergens (NDA) on pasteurized Akkermansia muciniphila as a novel food (NF) pursuant to Regulation (EU) 2015/2283: Safety of pasteurised Akkermansia muciniphila as a novel food pursuant to Regulation (EU) 2015/2283
Additional Resources:
Postbiotics. ISAPP infographic (also available in Japanese and Spanish).
Behind the publication: Understanding ISAPP’s new scientific consensus definition of postbiotics. ISAPP blog post.
Definition of postbiotics: A panel debate in Amsterdam. ISAPP blog post.
About Dr. Gabriel Vinderola:
Gabriel Vinderola graduated at the Faculty of Chemical Engineering from the National University of Litoral (Santa Fe, Argentina) in 1997. He obtained his Ph.D. in Chemistry in 2002 at the same University. He collaborated with several research teams in Canada, Spain, France, Italy, Germany, Brazil and Finland. He is presently Principal Researcher of the National Scientific and Technical Research Council (CONICET) and Associate Professor at the Food Engineering Department of his home Faculty. He participated in 1999 in the development of the first commercial cheese carrying probiotic bacteria in Latin America. In 2011, he was awarded the prize in Food Technology for young scientists, by the National Academy of Natural, Physic and Exact Sciences from Argentina. He published more than 120 original scientific publications in international refereed journals and book chapters. From 2020 to present, he serves as a member of the board of directors of the International Scientific Association for Probiotics and Prebiotcis (ISAPP). He is engaged in science communication to the general public through Instagram (@gvinde).
About Prof. Seppo Salminen:
Seppo Salminen, MSc, MS, PhD, is a Senior Advisor, Functional Foods Forum (FFF) at the University of Turku. His areas of expertise are gut microbiota, probiotics and prebiotics, nutrition and food safety, and EU regulations. Seppo teaches the topics of lactic acid biotechnology, functional foods and EU legislation and conducts research into food and health, intestinal microbiota, probiotics, prebiotics, functional foods, food legislation, health claims, and novel foods.