What’s a Clinician to do When the Probiotic Recommendations from Medical Organizations Do Not Agree?
By Prof. Hania Szajewska, MD, Department of Paediatrics, The Medical University of Warsaw, Poland
The scientific literature on probiotics is growing rapidly, with newly published studies continually adding to the sum of information about the probiotic strains that confer health benefits in specific populations.
In research, we make hypotheses. Eventually, they are resolved by collecting data or they are replaced by more refined, or entirely new, hypotheses. This process usually unfolds over an extended period of time. Along the way, medical and scientific organizations may decide to take ‘snapshots’ of the evidence to-date and develop guidelines based on available published studies. Unfortunately, disagreements can occur about the meaning of the data, sometimes leading to differences in the guidelines developed by various organizations.
But clinicians cannot always wait for the data to provide a crystal-clear picture. They want answers to guide their clinical practice. Hence the question: Should probiotics be used if guidelines do not agree on the use of probiotics for a certain indication, or on the strains to be used?
Take, for example, the current situation relevant to pediatric practice. Here I discuss two recommendation documents: one developed by the European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), and another developed by the American Gastroenterological Association (AGA).
Acute diarrhea
In 2020, the ESPGHAN Working Group (WG) on Probiotics identified 16 systematic reviews and meta-analyses published since 2010, which included more than 150 RCTs. The WG made weak (also known as conditional) recommendations for (in descending order in terms of the number of trials evaluating any given strain):
- S boulardii (low to very low certainty of evidence);
- L rhamnosus GG (very low certainty of evidence); L reuteri DSM 17938 (low to very low certainty of evidence);
- L rhamnosus 19070-2 & L reuteri DSM 12246 (very low certainty of evidence).
The WG made a strong recommendation against L helveticus R0052 & L rhamnosus R0011 (moderate certainty of evidence) and a weak (conditional) recommendation against Bacillus clausii strains O/C, SIN, N/R, and T (very low certainty of evidence)1.
In contrast, also in 2020, the AGA, based on the evaluation of 89 trials, made a conditional recommendation against the use of probiotics in children from North America with acute infectious gastroenteritis (moderate quality of evidence)2. The rationale for the negative AGA recommendation was that the majority of the studies were performed outside North America. Moreover, two large, high-quality null trials, performed in Canada and US, questioned the efficacy of the probiotics evaluated in these studies, for the management of children with acute gastroenteritis 3,4.
Prevention of necrotizing enterocolitis
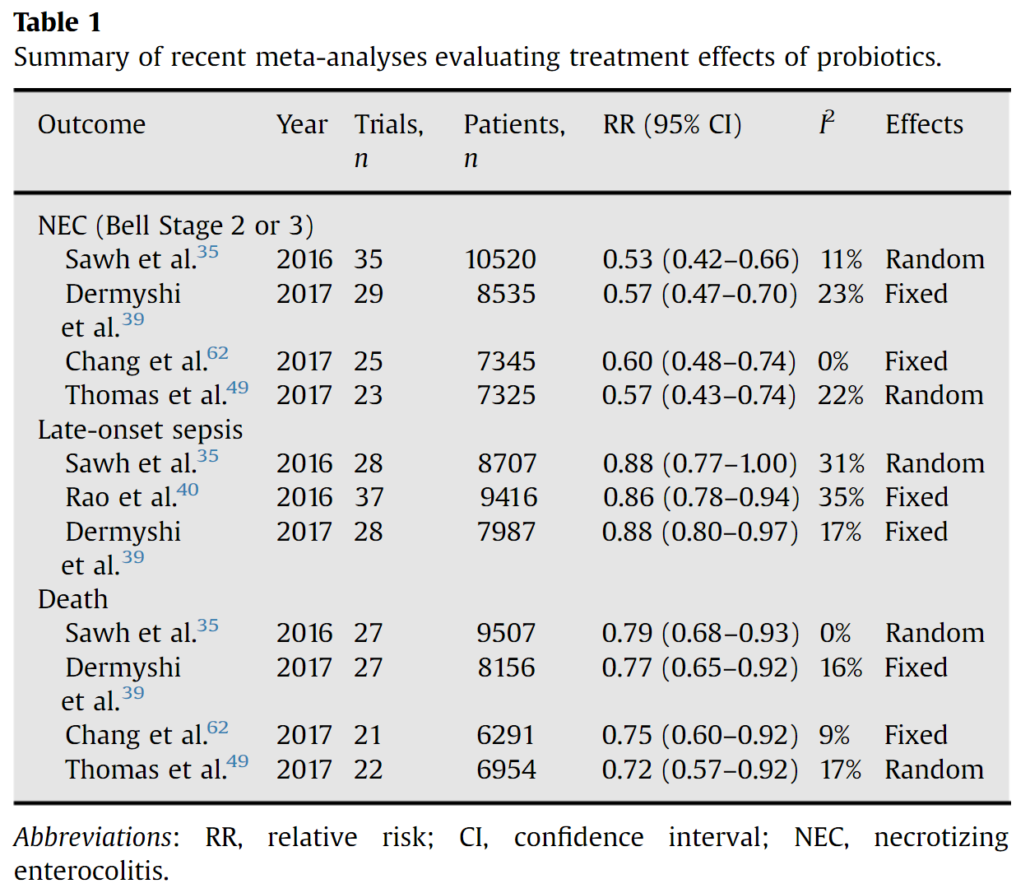
Another example of discordant guidelines relates to necrotizing enterocolitis (NEC) in preterm infants. NEC is one of the most severe and life-threatening gastrointestinal diseases to occur in preterm infants, particularly those with a birth weight <1,000 g. The factors involved in the pathogenesis of NEC include formula feeding rather than breastfeeding, intestinal hypoxia–ischemia, and colonization of the gut with pathogenic microbiota5.
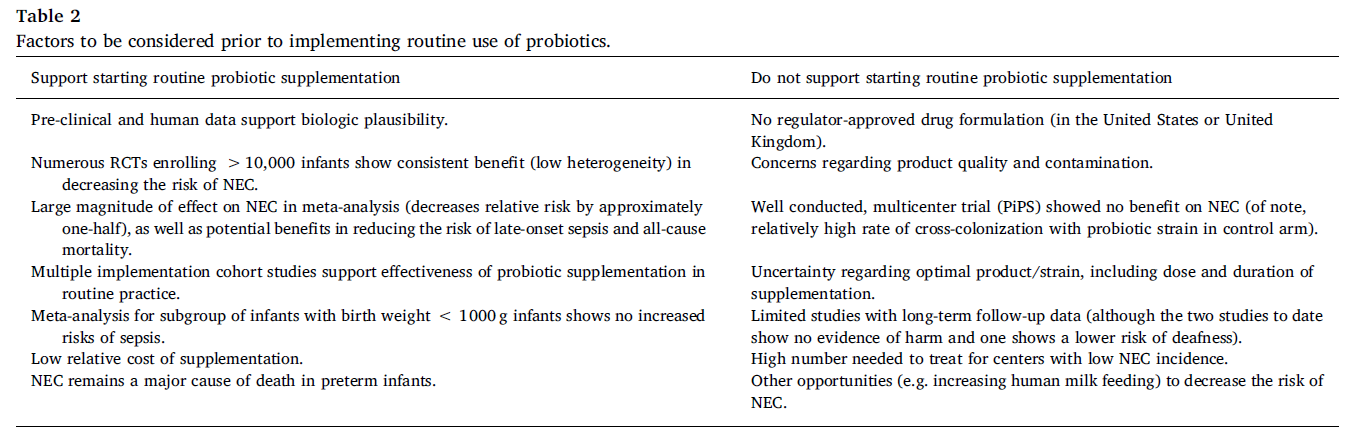
In 2020, both ESPGHAN6 and AGA2 published their recommendations on the use of probiotics for preventing NEC. While both were based on pair-wise systematic reviews and network meta-analyses7, their conclusions differed. The only probiotic strain that was recommended by both societies was L rhamnosus GG ATCC 53103. With regard to L reuteri DSM 17938, the ESPGHAN did not formulate a recommendation for or against it, while the AGA conditionally recommends it.
Why do guidelines differ?
Many factors contribute to the discrepancy in guidelines developed by various organizations. In the case of probiotics, they may be due to these differences:
- Study methods. Although dozens of studies involving thousands of patients have been conducted in many indications, studies are subject to bias resulting from incorrect randomization, non-confidentiality, non-masking, or lack of intention-to-treat analysis.
- Targeted population. The effectiveness of probiotics in different populations may vary, for example, due to differences diet or in microbiota at the start of treatment.
- Probiotics are a heterogeneous intervention. Even if the rules for assessing individual strains, and not probiotics as a group, are followed, the effectiveness of probiotics is influenced by factors such as product quality, storage conditions, dose, timing of administration, and the duration of the intervention.
- Outcome measures (endpoints). Studies use different outcomes to measure efficacy, and even if the same outcomes are used, their definition may differ (e.g. diarrhea duration may be defined as time to the last diarrheal stool or time to the first normal stool). Such heterogeneity in the reported outcomes, combined with the lack of standardized definitions, pose a challenge in meta-analyses and should be considered when interpreting the results.
What should clinicians do when the guidelines are not consistent?
Back to the question asked earlier: Should probiotics be routinely used if guidelines from the scientific or medical organizations do not agree on the use of probiotics?
One approach may not fit all. However, in the case of acute infectious diarrhea in children, both the AGA and ESPGHAN formulated a conditional recommendation: in the first case, it is negative; in the second, positive. It is important to note that the interpretation of a conditional recommendation for and a conditional recommendation against is similar. For clinicians, both mean that different choices will be appropriate for different people. Clinicians should help each patient make decisions consistent with the patient’s preferences. For patients, it means that the majority of individuals in this situation would want the suggested course of action, but many would not8.
Taken together, the recommendations communicate that probiotics may be beneficial, although not essential, in the treatment of acute diarrhea in young children. The use of certain probiotics with documented efficacy may be considered in the management of acute diarrhea in young children.
With regard to the prevention of NEC, the AGA and ESPGHAN guidelines agree that certain probiotics reduce the risk of NEC in preterm infants. However, based on their analyses and the included / excluded studies they differ in the recommended strains; additionally, not all of the strain combinations are available everywhere. Therefore, it seems reasonable to choose a probiotic that is included in the recommendations of both societies (if available). One example is L. rhamnosus GG.
In general, organizations should be commended for taking on the daunting task of evaluating the probiotic evidence – both the quality of the studies and the positive or negative results – in order to generate recommendations. Until further well-conducted studies make the answer clearer, clinicians must live with some ambiguity and use the recommendations in the best way possible to inform their daily decisions with individual patients.
REFERENCES
- Szajewska H, Guarino A, Hojsak I, et al. Use of Probiotics for the Management of Acute Gastroenteritis in Children. An Update. J Pediatr Gastroenterol Nutr. 2020.
- Su GL, Ko CW, Bercik P, et al. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology. 2020.
- Schnadower D, Tarr PI, Casper TC, et al. Lactobacillus rhamnosus GG versus Placebo for Acute Gastroenteritis in Children. The New England journal of medicine. 2018;379(21):2002-2014.
- Freedman SB, Williamson-Urquhart S, Farion KJ, et al. Multicenter Trial of a Combination Probiotic for Children with Gastroenteritis. The New England journal of medicine. 2018;379(21):2015-2026.
- Neu J, Walker WA. Necrotizing enterocolitis. The New England journal of medicine. 2011;364(3):255-264.
- van den Akker CHP, van Goudoever JB, Shamir R, et al. Probiotics and Preterm Infants: A Position Paper by the European Society for Paediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition Working Group for Probiotics and Prebiotics. Journal of pediatric gastroenterology and nutrition. 2020;70(5):664-680.
- van den Akker CHP, van Goudoever JB, Szajewska H, et al. Probiotics for Preterm Infants: A Strain-Specific Systematic Review and Network Meta-analysis. Journal of pediatric gastroenterology and nutrition. 2018;67(1):103-122.
- Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66(7):719-725.
See here for a published comment on this topic in The American Journal of Gastroenterology.