Lactobacillus bacteremia in critically ill patients does not raise questions about safety for general consumers
By Dan Merenstein MD, Professor of Family Medicine, Georgetown University Medical Center, Washington DC, USA; Eamonn Quigley MD, Professor of Medicine, Houston Methodist Hospital and Weill Cornell Medical College, Texas USA; Gregory Gloor PhD, Professor of Biochemistry, Schulich School of Medicine and Dentistry, The University of Western Ontario, Canada; Hania Szajewska MD, Professor of Paediatrics, The Medical University of Warsaw, Poland; and Mary Ellen Sanders PhD, Executive Science Officer, ISAPP, Colorado, USA.
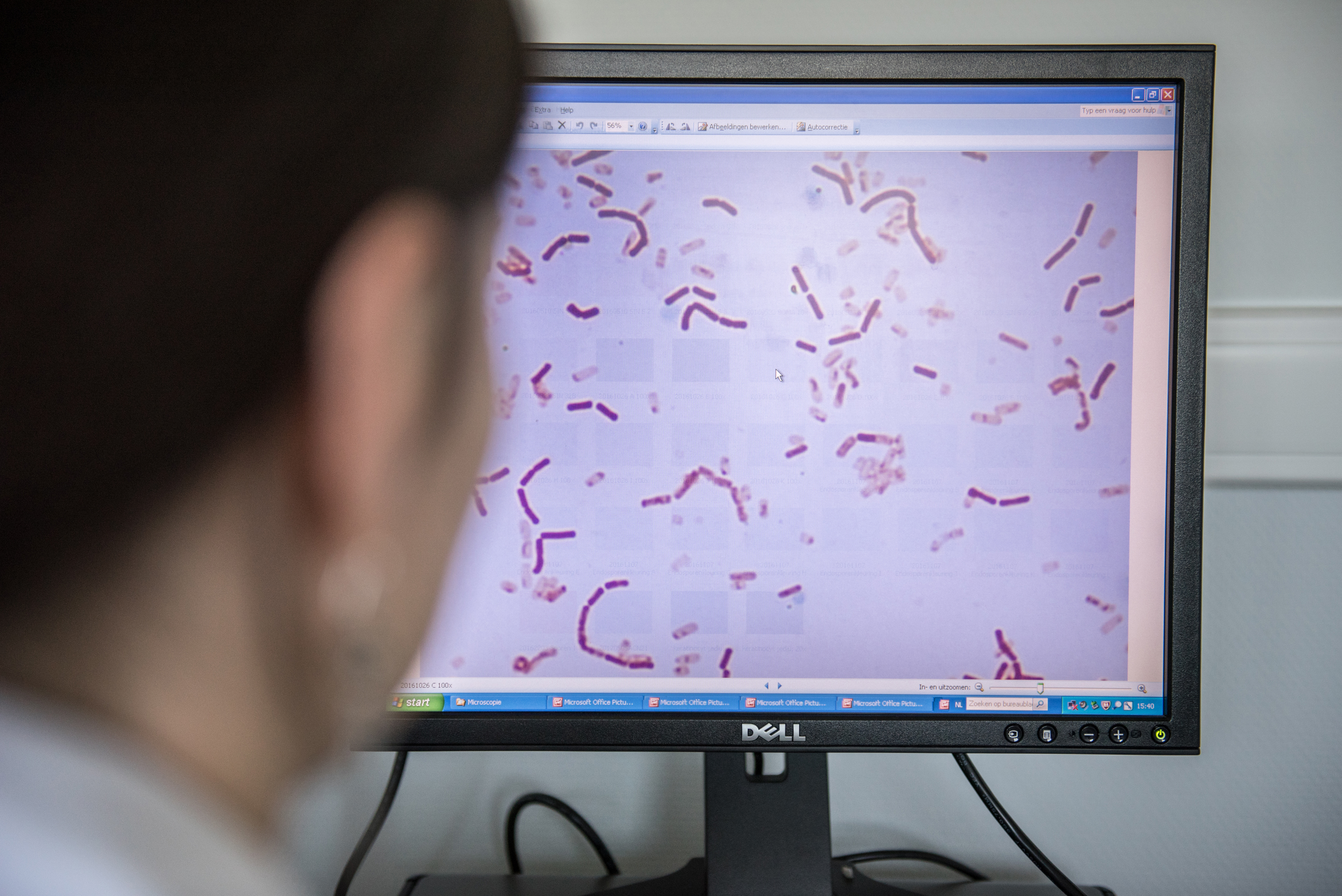
A recent Nature Medicine paper reported blood cultures positive for L. rhamnosus GG in six critically ill patients at Children’s Hospital in Boston.
About this study
Patients (aged 1, 2, 4, 12, 19 and 19 years) with L. rhamnosus-associated bacteremia suffered from different chronic conditions (mitochondrial disorder, cerebral palsy, congenital heart disease, cystic fibrosis) and were located either in the ICU (cardiac or medical/surgical ICU) or ICP (intermediate care program) at the hospital at the time of bacteremia. The bacteremia was discovered during routine blood culture screens. Clinical presentations were not described in detail; however, none had endocarditis or died from the bacteremia, although one did get a central line infection. Two of the six cases of bacteremia in probiotic-consuming patients were determined by attending physicians to be transient or due to contaminants, and were not treated. The other four cases were treated with antibiotics. A further 516 patients dosed with the same probiotic did not develop bacteremia.
The researchers examined the blood isolates and using whole genome sequencing were able to confirm that the Lactobacillus isolated from the blood of these patients was genetically identical – with the exception of a few SNPs – to L. rhamnosus GG present in the probiotic product. This is the preferred approach to confirming the source of blood culture isolates.
Important questions arising from critical review of this paper
- Was the study appropriately controlled?
The authors report a seemingly high rate (1.1%) of Lactobacillus bacteremia among the 522 L. rhamnosus-consuming patients compared with 0.009%, the rate of Lactobacillus bacteremia among 21,652 patients who did not receive probiotics. However, the paper does not justify the legitimacy of comparing these two groups to each other. Indeed, other underlying factors could contribute to the different rates of bacteremia, as these were not matched cohorts. It is important to recognize the limitations of the retrospective design used here, which limits the ability to match controls, and to control for cofounders such as underlying illness, severity of clinical illness and co-therapies (including antibiotics).
- What is the mechanism of transmission of the probiotic to the patients’ blood?
Most of the patients had a central line venous catheter. The paper reported that probiotics were mostly administered via tube feeding. If a probiotic is able to readily translocate the gut barrier in such patients, this would be a safety concern. But if the observed bacteremia was due to contamination of a central line, this may say more about hospital procedures than safety of the probiotic. Indeed, 16 years ago, central line contamination leading to fungemia was reported. In a 2005 paper, 92% of cases of fungemia associated with Saccharomyces cerevisiae var boulardii administration had an IV catheter. Based on such reports, handling dried probiotics in a hospital environment with critically ill patients should be done with caution. However, with proper administration procedures, certain probiotics are medically recommended in this setting.
- What was the clinical impact of administration of L. rhamnosus GG?
Important clinical parameters such as all-cause mortality (the outcome of greatest importance), length of hospital stay, abscesses, required medications, and others were not reported (although central line infection was reported) for the patients studied. The clinical context of this study would be more easily understood if information on the indications driving probiotic administration was provided. The authors question the risk/benefit of probiotic administration to ICU patients in a children’s hospital yet focus solely on risk and do not measure benefit. This suggests an underlying assumption by the authors that when it comes to probiotics, any risk is too much. Did the patients given L. rhamnosus GG suffer negative clinical outcomes more often than age and condition-matched controls? If so, then giving this probiotic to these patients cannot be recommended. But if not, then even though risk of bacteremia may be higher, if the patients given the probiotic fared better than matched patients, then the probiotic should be considered a reasonable option.
Lastly, the finding of a rate of Lactobacillus bacteremia of 1.1% needs to be viewed in the context of a 20% rate of nosocomial infections in the ICU (here and here).
Lessons regarding probiotic safety
Two main issues are raised by this study. The first is whether the evidence suggests opportunistic pathogenic properties of L. rhamnosus GG or rather that procedures used to administer probiotics in the ICU environment resulted in contamination, which caused bacteremia. No conclusions can be made from this study regarding this. The second is the importance of placing the results of this study into a clinical framework. The study implies risk from probiotic administration, even though the study was not powered for clinical outcomes and could not place any perceived increased risk into the context of any achieved benefit. Further, reporting rates of Lactobacillus bacteremia between cohorts unmatched for important characteristics except for probiotic use does not inform on relative risk.
Importantly for the broader situation of probiotic use, the ICU population is not reflective of the general population, so this study does not allow us to draw conclusions about safety of L. rhamnosus GG use in non-ICU patients.
We recognize the value of careful tracking of potential probiotic-associated infections and appreciate the application of bacterial genomic sequencing to identify the probiotic in the blood. Used more widely, this approach could resolve many purported claims of probiotic bacteremia.
This paper serves as an important reminder that use of probiotics in critically ill patients must be carefully considered and practice must align with learnings from the past, including the risk of central line contamination with probiotics. In addition, this paper highlights the importance of knowing the exact strain (including its antibiotic resistance profile and preferentially also its genome sequence), so that in the rare case of bacteremia, appropriate antibiotics can be administered.
See related article: Hill, C. Balancing the risks and rewards of live biotherapeutics. Nat Rev Gastroenterol Hepatol (2019)
See here for an additional related open-access publication: Probiotic use in at-risk populations. Sanders et al. 2014.