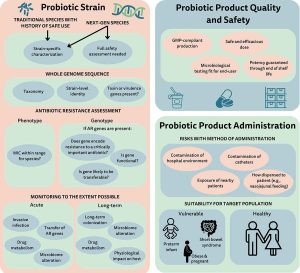
Considerations for determining safety of probiotics: A USP perspective
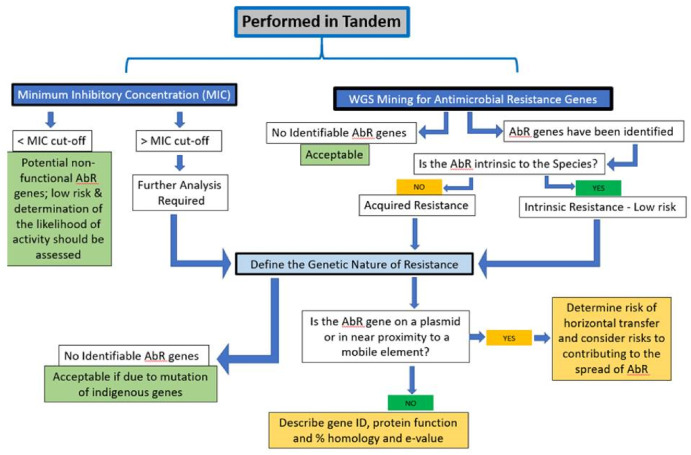
Several stakeholders, including the United States Pharmacopeia (USP), have an interest in developing best practice guidelines for assessing the quality and safety of probiotics. This review provides a summary guide to global regulatory guidelines and outlines the essential parameters of a comprehensive safety assessment for a probiotic product.
REFERENCE:
Roe AL, Boyte ME, Elkins CA, Goldman VS, Heimbach J, Madden E, Oketch-Rabah H, Sanders ME, Sirois J, Smith A. Considerations for determining safety of probiotics: A USP perspective. Regul Toxicol Pharmacol 2022; 136:105266. doi: 10.1016/j.yrtph.2022.105266.